Inflammatory networks during cellular senescence: causes and consequences - PubMed (original) (raw)
Review
Inflammatory networks during cellular senescence: causes and consequences
Adam Freund et al. Trends Mol Med. 2010 May.
Abstract
Chronic inflammation is associated with aging and plays a causative role in several age-related diseases such as cancer, atherosclerosis and osteoarthritis. The source of this chronic inflammation is often attributed to the progressive activation of immune cells over time. However, recent studies have shown that the process of cellular senescence, a tumor suppressive stress response that is also associated with aging, entails a striking increase in the secretion of proinflammatory proteins and might be an important additional contributor to chronic inflammation. Here, we list the secreted factors that make up the proinflammatory phenotype of senescent cells and describe the impact of these factors on tissue homeostasis. We also summarize the cellular pathways/processes that are known to regulate this phenotype--namely, the DNA damage response, microRNAs, key transcription factors and kinases and chromatin remodeling.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
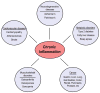
Figure 1. Chronic inflammation is associated with most age-related diseases
There is an extensive range of conditions and diseases that are associated with chronic inflammation or that have an inflammatory component. Chronic inflammation lies at the root of heart disease, cancer, osteoporosis, Alzheimer’s disease, diabetes and many other age-related diseases.
Figure 2. Effects of the SASP on tissue homeostasis
The response of cells to the SASP depends on cell type and cell context. The SASP affects the original senescent cell by stimulating clearance by NK cells and reinforcing the senescence growth arrest. The SASP affects surrounding non-immune cells as well; it increases the proliferation of nearby epithelial and stromal cells, promotes invasion of any nearby preneoplastic or neoplastic cells via an epithelial to mesenchymal transition, stimulates angiogenesis by stimulating endothelial cell migration and invasion, and disrupts normal tissue structures and function.
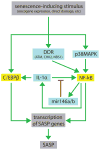
Figure 3. Pathways that regulate the SASP
A senescence-inducing stimulus causes genotoxic stress (e.g. DNA damage or chromatin unwinding), which activates the DDR and p38MAPK pathways. These pathways cooperate to activate NF-κB. C/EBPβ is activated through an unknown mechanism. NF-κB and C/EBPβ activate the transcription of SASP genes. IL-1α, which is regulated by NF-κB, further increases NF-κB activity via a positive feedback loop; this loop is dampened by mir146a/b, which are also induced by NF-κB. This complex regulatory network increases the expression of pro-inflammatory factors, which are then secreted. Green arrows indicate activation of the SASP; red lines indicate inhibition of the SASP; blue boxes are upstream signaling components; yellow boxes are transcription factors.
Similar articles
- Senescent cells as a source of inflammatory factors for tumor progression.
Davalos AR, Coppe JP, Campisi J, Desprez PY. Davalos AR, et al. Cancer Metastasis Rev. 2010 Jun;29(2):273-83. doi: 10.1007/s10555-010-9220-9. Cancer Metastasis Rev. 2010. PMID: 20390322 Free PMC article. Review. - MicroRNAs linking inflamm-aging, cellular senescence and cancer.
Olivieri F, Rippo MR, Monsurrò V, Salvioli S, Capri M, Procopio AD, Franceschi C. Olivieri F, et al. Ageing Res Rev. 2013 Sep;12(4):1056-68. doi: 10.1016/j.arr.2013.05.001. Epub 2013 May 17. Ageing Res Rev. 2013. PMID: 23688930 Review. - Senescent Vascular Smooth Muscle Cells Drive Inflammation Through an Interleukin-1α-Dependent Senescence-Associated Secretory Phenotype.
Gardner SE, Humphry M, Bennett MR, Clarke MC. Gardner SE, et al. Arterioscler Thromb Vasc Biol. 2015 Sep;35(9):1963-74. doi: 10.1161/ATVBAHA.115.305896. Epub 2015 Jul 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 26139463 Free PMC article. - Senescent endothelial cells: Potential modulators of immunosenescence and ageing.
Pantsulaia Ia, Ciszewski WM, Niewiarowska J. Pantsulaia Ia, et al. Ageing Res Rev. 2016 Aug;29:13-25. doi: 10.1016/j.arr.2016.05.011. Epub 2016 May 26. Ageing Res Rev. 2016. PMID: 27235855 Review. - Roles and mechanisms of cellular senescence in regulation of tissue homeostasis.
Ohtani N, Hara E. Ohtani N, et al. Cancer Sci. 2013 May;104(5):525-30. doi: 10.1111/cas.12118. Epub 2013 Mar 11. Cancer Sci. 2013. PMID: 23360516 Free PMC article. Review.
Cited by
- Metformin improves tendon degeneration by blocking translocation of HMGB1 and suppressing tendon inflammation and senescence in aging mice.
Zhang J, Brown R, Hogan MV, Onishi K, Wang JH. Zhang J, et al. J Orthop Res. 2023 Jun;41(6):1162-1176. doi: 10.1002/jor.25470. Epub 2022 Nov 7. J Orthop Res. 2023. PMID: 36262012 Free PMC article. - A non-canonical role for a small nucleolar RNA in ribosome biogenesis and senescence.
Cheng Y, Wang S, Zhang H, Lee JS, Ni C, Guo J, Chen E, Wang S, Acharya A, Chang TC, Buszczak M, Zhu H, Mendell JT. Cheng Y, et al. Cell. 2024 Aug 22;187(17):4770-4789.e23. doi: 10.1016/j.cell.2024.06.019. Epub 2024 Jul 8. Cell. 2024. PMID: 38981482 - The challenge of prognostic markers in acute pancreatitis: internist's point of view.
Para O, Caruso L, Savo MT, Antonielli E, Blasi E, Capello F, Ciarambino T, Corbo L, Curto A, Giampieri M, Maddaluni L, Zaccagnini G, Nozzoli C. Para O, et al. J Genet Eng Biotechnol. 2021 May 25;19(1):77. doi: 10.1186/s43141-021-00178-3. J Genet Eng Biotechnol. 2021. PMID: 34036463 Free PMC article. - Role of Hyperglycemia in the Senescence of Mesenchymal Stem Cells.
Yin M, Zhang Y, Yu H, Li X. Yin M, et al. Front Cell Dev Biol. 2021 Apr 15;9:665412. doi: 10.3389/fcell.2021.665412. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33968939 Free PMC article. Review. - Late-life rapamycin treatment reverses age-related heart dysfunction.
Flynn JM, O'Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S. Flynn JM, et al. Aging Cell. 2013 Oct;12(5):851-62. doi: 10.1111/acel.12109. Epub 2013 Jul 7. Aging Cell. 2013. PMID: 23734717 Free PMC article.
References
- Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236(1):13–23. - PubMed
- Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–52. - PubMed
- Vasto S, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. - PubMed
- Ferrucci L, et al. A flame burning within. Aging Clin Exp Res. 2004;16(3):240–3. - PubMed
- Bruunsgaard H. The clinical impact of systemic low-level inflammation in elderly populations. With special reference to cardiovascular disease, dementia and mortality. Dan Med Bull. 2006;53(3):285–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG025901/AG/NIA NIH HHS/United States
- RL1 AG032117/AG/NIA NIH HHS/United States
- T32 AG000266/AG/NIA NIH HHS/United States
- AG09909/AG/NIA NIH HHS/United States
- R37 AG009909/AG/NIA NIH HHS/United States
- R56 AG009909/AG/NIA NIH HHS/United States
- AG032117/AG/NIA NIH HHS/United States
- P01 AG017242/AG/NIA NIH HHS/United States
- P30 AG025708/AG/NIA NIH HHS/United States
- AG025901/AG/NIA NIH HHS/United States
- AG017242/AG/NIA NIH HHS/United States
- AG025708/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources