Microvesicles: mediators of extracellular communication during cancer progression - PubMed (original) (raw)
Review
Microvesicles: mediators of extracellular communication during cancer progression
Vandhana Muralidharan-Chari et al. J Cell Sci. 2010.
Abstract
Microvesicles are generated by the outward budding and fission of membrane vesicles from the cell surface. Recent studies suggest that microvesicle shedding is a highly regulated process that occurs in a spectrum of cell types and, more frequently, in tumor cells. Microvesicles have been widely detected in various biological fluids including peripheral blood, urine and ascitic fluids, and their function and composition depend on the cells from which they originate. By facilitating the horizontal transfer of bioactive molecules such as proteins, RNAs and microRNAs, they are now thought to have vital roles in tumor invasion and metastases, inflammation, coagulation, and stem-cell renewal and expansion. This Commentary summarizes recent literature on the properties and biogenesis of microvesicles and their potential role in cancer progression.
Figures
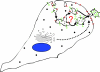
Fig. 1.
Microvesicle shedding. Microvesicles are formed by the outward budding of the plasma membrane, as shown. Not all plasma-membrane proteins are incorporated into shed vesicles, although the topology of membrane proteins remains intact. Membrane proteins such as oncogene and other growth-factor receptors, intergrin receptors and MHC class I molecules, soluble proteins such as proteases and cytokines, as well as nucleic acids, have been found in microvesicles. Microvesicles appear to be enriched in some lipids such as cholesterol, whereas phosphatidylserine is relocated to the outer membrane leaflet specifically at sites of microvesicle shedding.
Fig. 2.
Working model for the trafficking of cargo to sites of microvesicle shedding. Recent studies suggest that a variety of proteins, including MHC class I, β1 intergrin receptors and VAMP3 – which are trafficked via specialized early recycling endosomes – are selectively incorporated into microvesicles. It is unclear whether cargo sorting occurs in endosomes so that pre-packaged vesicles are trafficked to the cell surface, or whether sorting occurs at the plasma membrane. Proteins trafficked via other pathways (dashed line) could also be delivered to microvesicles.
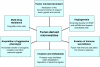
Fig. 3.
Tumor-derived microvesicles influence many aspects of cancer progression. By their ability to harness select bioactive molecules and propagate the horizontal transfer of these cargoes, tumor-derived microvesicles can affect a variety of cellular events to have an enormous impact on tumor progression.
Similar articles
- Microvesicles: messengers and mediators of tumor progression.
Al-Nedawi K, Meehan B, Rak J. Al-Nedawi K, et al. Cell Cycle. 2009 Jul 1;8(13):2014-8. doi: 10.4161/cc.8.13.8988. Epub 2009 Jul 11. Cell Cycle. 2009. PMID: 19535896 Review. - Deciphering the role of ectosomes in cancer development and progression: focus on the proteome.
Surman M, Stępień E, Hoja-Łukowicz D, Przybyło M. Surman M, et al. Clin Exp Metastasis. 2017 Apr;34(3-4):273-289. doi: 10.1007/s10585-017-9844-z. Epub 2017 Mar 19. Clin Exp Metastasis. 2017. PMID: 28317069 Free PMC article. Review. - Microvesicles as mediators of intercellular communication in cancer.
Antonyak MA, Cerione RA. Antonyak MA, et al. Methods Mol Biol. 2014;1165:147-73. doi: 10.1007/978-1-4939-0856-1_11. Methods Mol Biol. 2014. PMID: 24839024 Review. - Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer.
Sheehan C, D'Souza-Schorey C. Sheehan C, et al. J Cell Sci. 2019 Oct 15;132(20):jcs235085. doi: 10.1242/jcs.235085. J Cell Sci. 2019. PMID: 31615844 Free PMC article. Review. - Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers.
D'Souza-Schorey C, Clancy JW. D'Souza-Schorey C, et al. Genes Dev. 2012 Jun 15;26(12):1287-99. doi: 10.1101/gad.192351.112. Genes Dev. 2012. PMID: 22713869 Free PMC article. Review.
Cited by
- Harmonising cellular conversations: decoding the vital roles of extracellular vesicles in respiratory system intercellular communications.
Jadamba B, Jin Y, Lee H. Jadamba B, et al. Eur Respir Rev. 2024 Nov 13;33(174):230272. doi: 10.1183/16000617.0272-2023. Print 2024 Oct. Eur Respir Rev. 2024. PMID: 39537245 Free PMC article. Review. - Synthetic Peptides Induce Human Colorectal Cancer Cell Death via Proapoptotic Pathways.
Mesquita FP, de Oliveira FL, da Silva EL, Brito DMS, de Moraes MEA, Souza PFN, Montenegro RC. Mesquita FP, et al. ACS Omega. 2024 Oct 11;9(42):43252-43263. doi: 10.1021/acsomega.4c08194. eCollection 2024 Oct 22. ACS Omega. 2024. PMID: 39464451 Free PMC article. - Perivascular Adipose Tissue and Perivascular Adipose Tissue-Derived Extracellular Vesicles: New Insights in Vascular Disease.
Sigdel S, Udoh G, Albalawy R, Wang J. Sigdel S, et al. Cells. 2024 Aug 6;13(16):1309. doi: 10.3390/cells13161309. Cells. 2024. PMID: 39195199 Free PMC article. Review. - Unraveling the Connection: Extracellular Vesicles and Non-Small Cell Lung Cancer.
Wu J, Chen Y. Wu J, et al. Int J Nanomedicine. 2024 Aug 9;19:8139-8157. doi: 10.2147/IJN.S477851. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39139506 Free PMC article. Review.
References
- Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619-624 - PubMed
- Anderson H. C., Garimella R., Tague S. E. (2005). The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 10, 822-837 - PubMed
- Angelucci A., D'Ascenzo S., Festuccia C., Gravina G. L., Bologna M., Dolo V., Pavan A. (2000). Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin. Exp. Metastasis 18, 163-170 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources