Blood-borne amyloid-beta dimer correlates with clinical markers of Alzheimer's disease - PubMed (original) (raw)
. 2010 May 5;30(18):6315-22.
doi: 10.1523/JNEUROSCI.5180-09.2010.
Keyla A Perez, Kerryn E Pike, W Mei Kok, Christopher C Rowe, Anthony R White, Pierrick Bourgeat, Olivier Salvado, Justin Bedo, Craig A Hutton, Noel G Faux, Colin L Masters, Kevin J Barnham
Affiliations
- PMID: 20445057
- PMCID: PMC6632738
- DOI: 10.1523/JNEUROSCI.5180-09.2010
Blood-borne amyloid-beta dimer correlates with clinical markers of Alzheimer's disease
Victor L Villemagne et al. J Neurosci. 2010.
Abstract
Alzheimer's disease (AD) is the most common age-related dementia. Unfortunately due to a lack of validated biomarkers definitive diagnosis relies on the histological demonstration of amyloid-beta (Abeta) plaques and tau neurofibrillary tangles. Abeta processing is implicated in AD progression and many therapeutic strategies target various aspects of this biology. While Abeta deposition is the most prominent feature of AD, oligomeric forms of Abeta have been implicated as the toxic species inducing the neuronal dysfunction. Currently there are no methods allowing routine monitoring of levels of such species in living populations. We have used surface enhanced laser desorption ionization time of flight (SELDI-TOF) mass spectrometry incorporating antibody capture to investigate whether the cellular membrane-containing fraction of blood provides a new source of biomarkers. There are significant differences in the mass spectra profiles of AD compared with HC subjects, with significantly higher levels of Abeta monomer and dimer in the blood of AD subjects. Furthermore, levels of these species correlated with clinical markers of AD including brain Abeta burden, cognitive impairment and brain atrophy. These results indicate that fundamental biochemical events relevant to AD can be monitored in blood, and that the species detected may be useful clinical biomarkers for AD.
Figures
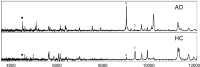
Figure 1.
Representative SELDI-TOF/MS spectra. Samples extracted from the CE of blood from an AD subject (top) and a normal age-matched control (bottom). In these examples the antibody WO2 was used. Peaks marked with • are Aβ42 and the corresponding dimer (*), respectively, are elevated in AD. In contrast, the 9962 Da peak (†) is elevated in HC.
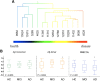
Figure 2.
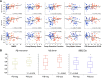
Distribution of blood SELDI-TOF MS values in regards to mass to charge ratio and clinical classification in 118 participants. Hierarchical cluster analysis showing two distinct classes of peaks associated with the normal or abnormal processing of APP. A, At the left end, peak 9962 Da, a species that was higher in HC than in AD. On the right end of the cluster are peaks due to the Aβ monomer and dimer that were higher in AD. The pattern of the cluster analysis is consistent with two different processing pathways for APP: an amyloidogenic pathway that is elevated in AD and a non-amyloidogenic pathway that is elevated in the control subjects. Box-and-whiskers plots comparing the intensities of Aβ monomer, dimer, and the peak at 9962 Da in HC, MCI, and AD subjects. B, A good separation is observed between the AD and HC groups (Cohen's d: 0.41, 0.76 and 0.73 for monomer, dimer and 9962 Da peak, respectively).
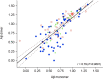
Figure 3.
Correlation between Aβ42 monomer and dimer. The intensities of the peaks assigned to the monomeric Aβ42 and the corresponding dimer from 118 participants are highly correlated (r = 0.79, p < 0.0001). HC, Blue circle; MCI, green +; AD, red circle.
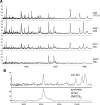
Figure 4.
SELDI-TOF MS of CEs from an AD subject. A, Peaks due to monomeric (*) and dimeric (**) Aβ42 are detected by three different antibodies: WO2 (epitope 4–8), 4G8 (epitope 17–21), and G211 (C terminus of Aβ42), but not by G210 (C terminus of Aβ40). B, Comparison of SELDI-TOF MS profile of the Aβ dimer detected in the CEs with that of a synthetic Aβ dimer in which the Aβ peptide chains contain a sulfoxide at residue M35 and are covalently crosslinked by a dityrosine moiety.
Figure 5.
Relationship between blood SELDI-TOF MS mass to charge ratios and clinical and neuroimaging parameters. Aβ monomer, dimer and 9962 Da are highly correlated with clinical, neuropsychometric and biological markers, such as MMSE, memory performance, executive function, gray matter volume, and brain Aβ burden as measured by PiB-PET, underlying their interrelationship. A, These graphs reflect the balance in APP processing between the amyloidogenic and non-amyloidogenic pathway that defines AD. HC, Blue circle; MCI, green +; AD, red circle. Box-and-whiskers plots of the intensities of Aβ monomer, dimer, and 9962 with brain Aβ burden as measured by PiB PET. B, A PiB SUVR threshold of 1.45 was used to separate the groups in PiB-positive (PiB-pos) and PiB-negative (PiB-neg).
Figure 6.
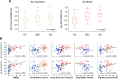
Characteristics and relationship of the ratio of the blood SELDI-TOF MS values with clinical and neuroimaging parameters in 118 participants. Box-and-whiskers plots comparing the respective Aβ monomer and dimer to 9962 Da ratios in HC, MCI, and AD subjects. A, A much better separation between the AD and HC groups is obtained (Cohen's d: 0.76 and 1.03 for monomer and dimer ratios, respectively) than when the peak intensities are examined separately. Aβ monomer and dimer to 9962 Da ratios are highly correlated with clinical, neuropsychometric, and biological markers. B, The correlations are better than when either the monomer, the dimer, or the 9962 Da peaks are examined separately. HC, Blue circle; MCI, green +; AD, red circle.
Similar articles
- Increasing the predictive accuracy of amyloid-β blood-borne biomarkers in Alzheimer's disease.
Watt AD, Perez KA, Faux NG, Pike KE, Rowe CC, Bourgeat P, Salvado O, Masters CL, Villemagne VL, Barnham KJ. Watt AD, et al. J Alzheimers Dis. 2011;24(1):47-59. doi: 10.3233/JAD-2010-101722. J Alzheimers Dis. 2011. PMID: 21157020 - A New Serum Biomarker Set to Detect Mild Cognitive Impairment and Alzheimer's Disease by Peptidome Technology.
Abe K, Shang J, Shi X, Yamashita T, Hishikawa N, Takemoto M, Morihara R, Nakano Y, Ohta Y, Deguchi K, Ikeda M, Ikeda Y, Okamoto K, Shoji M, Takatama M, Kojo M, Kuroda T, Ono K, Kimura N, Matsubara E, Osakada Y, Wakutani Y, Takao Y, Higashi Y, Asada K, Senga T, Lee LJ, Tanaka K. Abe K, et al. J Alzheimers Dis. 2020;73(1):217-227. doi: 10.3233/JAD-191016. J Alzheimers Dis. 2020. PMID: 31771070 Free PMC article. - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- Soluble Aβ oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes.
Hong S, Ostaszewski BL, Yang T, O'Malley TT, Jin M, Yanagisawa K, Li S, Bartels T, Selkoe DJ. Hong S, et al. Neuron. 2014 Apr 16;82(2):308-19. doi: 10.1016/j.neuron.2014.02.027. Epub 2014 Mar 27. Neuron. 2014. PMID: 24685176 Free PMC article. - Time-dependent changes in gene expression induced by secreted amyloid precursor protein-alpha in the rat hippocampus.
Ryan MM, Morris GP, Mockett BG, Bourne K, Abraham WC, Tate WP, Williams JM. Ryan MM, et al. BMC Genomics. 2013 Jun 6;14:376. doi: 10.1186/1471-2164-14-376. BMC Genomics. 2013. PMID: 23742273 Free PMC article. - Human anti-Aβ IgGs target conformational epitopes on synthetic dimer assemblies and the AD brain-derived peptide.
Welzel AT, Williams AD, McWilliams-Koeppen HP, Acero L, Weber A, Blinder V, Mably A, Bunk S, Hermann C, Farrell MA, Ehrlich HJ, Schwarz HP, Walsh DM, Solomon A, O'Nuallain B. Welzel AT, et al. PLoS One. 2012;7(11):e50317. doi: 10.1371/journal.pone.0050317. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209707 Free PMC article. - Mechanisms of neural and behavioral dysfunction in Alzheimer's disease.
Wesson DW, Nixon RA, Levy E, Wilson DA. Wesson DW, et al. Mol Neurobiol. 2011 Jun;43(3):163-79. doi: 10.1007/s12035-011-8177-1. Epub 2011 Mar 22. Mol Neurobiol. 2011. PMID: 21424679 Free PMC article. Review. - Amyloid-β oligomers stimulate microglia through a tyrosine kinase dependent mechanism.
Dhawan G, Floden AM, Combs CK. Dhawan G, et al. Neurobiol Aging. 2012 Oct;33(10):2247-61. doi: 10.1016/j.neurobiolaging.2011.10.027. Epub 2011 Dec 1. Neurobiol Aging. 2012. PMID: 22133278 Free PMC article.
References
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, et al. Rapid restoration of cognition in Alzheimer's transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. - PubMed
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. - PMC - PubMed
- Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling KQ, Huang X, Moir RD, Wang D, Sayre LM, Smith MA, Chen SG, Bush AI. Copper mediates dityrosine cross-linking of Alzheimer's amyloid-beta. Biochemistry. 2004;43:560–568. - PubMed
- Barnham KJ, Ciccotosto GD, Tickler AK, Ali FE, Smith DG, Williamson NA, Lam YH, Carrington D, Tew D, Kocak G, Volitakis I, Separovic F, Barrow CJ, Wade JD, Masters CL, Cherny RA, Curtain CC, Bush AI, Cappai R. Neurotoxic, redox-competent Alzheimer's beta-amyloid is released from lipid membrane by methionine oxidation. J Biol Chem. 2003;278:42959–42965. - PubMed
- Barnham KJ, Haeffner F, Ciccotosto GD, Curtain CC, Tew D, Mavros C, Beyreuther K, Carrington D, Masters CL, Cherny RA, Cappai R, Bush AI. Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer's disease beta-amyloid. FASEB J. 2004;18:1427–1429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical