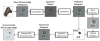Lensfree microscopy on a cellphone - PubMed (original) (raw)
. 2010 Jul 21;10(14):1787-92.
doi: 10.1039/c003477k. Epub 2010 May 6.
Affiliations
- PMID: 20445943
- PMCID: PMC2941438
- DOI: 10.1039/c003477k
Lensfree microscopy on a cellphone
Derek Tseng et al. Lab Chip. 2010.
Abstract
We demonstrate lensfree digital microscopy on a cellphone. This compact and light-weight holographic microscope installed on a cellphone does not utilize any lenses, lasers or other bulky optical components and it may offer a cost-effective tool for telemedicine applications to address various global health challenges. Weighing approximately 38 grams (<1.4 ounces), this lensfree imaging platform can be mechanically attached to the camera unit of a cellphone where the samples are loaded from the side, and are vertically illuminated by a simple light-emitting diode (LED). This incoherent LED light is then scattered from each micro-object to coherently interfere with the background light, creating the lensfree hologram of each object on the detector array of the cellphone. These holographic signatures captured by the cellphone permit reconstruction of microscopic images of the objects through rapid digital processing. We report the performance of this lensfree cellphone microscope by imaging various sized micro-particles, as well as red blood cells, white blood cells, platelets and a waterborne parasite (Giardia lamblia).
Figures
Fig. 1
(a) A lensfree cellphone microscope which operates based on incoherent in-line holography is shown. The additional hardware installed on the cellphone weighs ~38 grams (<1.4 ounces) and is composed of an inexpensive light emitting diode (at 587 nm) with an aperture of ~100 μm in front of the source. This cellphone microscope does not utilize any lenses or other bulky optical components and operates with a unit fringe magnification to claim the entire active area of the sensor as its imaging field of view. The samples to be imaged are loaded from the side through a mechanical sample holder. (b) Schematic diagram of the microscope attachment shown in (a) is illustrated.
Fig. 2
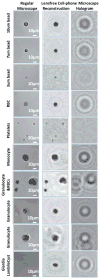
(a) Imaging performance of the lensfree cellphone microscope shown in Fig. 1a is compared against a regular microscope (10× objective lens, 0.25 numerical aperture) for various micro-objects, including red blood cells, white blood cells (monocytes and granulocytes), platelets, Giardia lamblia cyst, as well as 3, 7 and 10 μm diameter particles. The lensfree holograms captured by the cellphone sensor are digitally processed within less than 30 ms to reconstruct microscopic images of the specimen as shown on the middle column.
Fig. 3
The change in the reconstructed image quality of the lensfree cellphone microscope is illustrated as a function of the number of steps used for uniform quantization. The objects are red blood cells and a granulocyte on a blood smear sample. The top row presents the processed lensfree holograms of the cells captured by our cellphone microscope, where the digital size (in kBytes, when saved in PNG format) of each holographic image is indicated at the left corner as an inset. The middle row presents the reconstructed images of the cells for each bit depth. These results demonstrate that even for a bit depth of 4 (the second column on the right), the holographic recovery still remains very good at an average image size of 2.94 Bits/Pixel. This implies that for one pixel of the holographic image it would only be necessary to transmit (on average) 2.94 Bits in PNG format. In other words, 1 Mega Pixel worth of holographic data (corresponding to an imaging field of view of ~5 mm2) on average would require transmission of only ~0.38 Mbytes.
Fig. 4
Our de-Bayering algorithm developed to create monochrome holographic images from Bayer patterned output of our lensfree cell-phone microscope is summarized. Red and green channels of the acquired raw holographic image are equalized using a background image that was recorded with identical illumination conditions as the object. Blue pixels are estimated from their red and green neighbors (which include high SNR information) using an edge-aware interpolation approach and are further refined through an iterative recovery process with the help of an automatically generated object support mask. Finally, the recovered hologram is up-sampled and fed into a custom-developed holographic reconstruction algorithm, to create the corresponding microscopic images of the objects.
Similar articles
- Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications.
Mudanyali O, Tseng D, Oh C, Isikman SO, Sencan I, Bishara W, Oztoprak C, Seo S, Khademhosseini B, Ozcan A. Mudanyali O, et al. Lab Chip. 2010 Jun 7;10(11):1417-28. doi: 10.1039/c000453g. Epub 2010 Apr 19. Lab Chip. 2010. PMID: 20401422 Free PMC article. - Smart-phone based computational microscopy using multi-frame contact imaging on a fiber-optic array.
Navruz I, Coskun AF, Wong J, Mohammad S, Tseng D, Nagi R, Phillips S, Ozcan A. Navruz I, et al. Lab Chip. 2013 Oct 21;13(20):4015-23. doi: 10.1039/c3lc50589h. Epub 2013 Aug 12. Lab Chip. 2013. PMID: 23939637 Free PMC article. - Lensfree on-chip microscopy over a wide field-of-view using pixel super-resolution.
Bishara W, Su TW, Coskun AF, Ozcan A. Bishara W, et al. Opt Express. 2010 May 24;18(11):11181-91. doi: 10.1364/OE.18.011181. Opt Express. 2010. PMID: 20588977 Free PMC article. - Lensfree optofluidic microscopy and tomography.
Bishara W, Isikman SO, Ozcan A. Bishara W, et al. Ann Biomed Eng. 2012 Feb;40(2):251-62. doi: 10.1007/s10439-011-0385-3. Epub 2011 Sep 2. Ann Biomed Eng. 2012. PMID: 21887590 Review. - Partially coherent lensfree tomographic microscopy [Invited].
Isikman SO, Bishara W, Ozcan A. Isikman SO, et al. Appl Opt. 2011 Dec 1;50(34):H253-64. doi: 10.1364/AO.50.00H253. Appl Opt. 2011. PMID: 22193016 Free PMC article. Review.
Cited by
- Capillary-Driven Flow Microfluidics Combined with Smartphone Detection: An Emerging Tool for Point-of-Care Diagnostics.
Hassan SU, Tariq A, Noreen Z, Donia A, Zaidi SZJ, Bokhari H, Zhang X. Hassan SU, et al. Diagnostics (Basel). 2020 Jul 22;10(8):509. doi: 10.3390/diagnostics10080509. Diagnostics (Basel). 2020. PMID: 32708045 Free PMC article. Review. - Improving the Sensitivity and Functionality of Mobile Webcam-Based Fluorescence Detectors for Point-of-Care Diagnostics in Global Health.
Rasooly R, Bruck HA, Balsam J, Prickril B, Ossandon M, Rasooly A. Rasooly R, et al. Diagnostics (Basel). 2016 May 17;6(2):19. doi: 10.3390/diagnostics6020019. Diagnostics (Basel). 2016. PMID: 27196933 Free PMC article. Review. - High-Precision Lens-Less Flow Cytometer on a Chip.
Fang Y, Yu N, Jiang Y, Dang C. Fang Y, et al. Micromachines (Basel). 2018 May 10;9(5):227. doi: 10.3390/mi9050227. Micromachines (Basel). 2018. PMID: 30424160 Free PMC article. - Wide-field optical detection of nanoparticles using on-chip microscopy and self-assembled nanolenses.
Mudanyali O, McLeod E, Luo W, Greenbaum A, Coskun AF, Hennequin Y, Allier CP, Ozcan A. Mudanyali O, et al. Nat Photonics. 2013 Mar 1;7(3):10.1038/nphoton.2012.337. doi: 10.1038/nphoton.2012.337. Nat Photonics. 2013. PMID: 24358054 Free PMC article. - Quantitative, high-sensitivity measurement of liquid analytes using a smartphone compass.
Ferris M, Zabow G. Ferris M, et al. Nat Commun. 2024 Mar 30;15(1):2801. doi: 10.1038/s41467-024-47073-2. Nat Commun. 2024. PMID: 38555368 Free PMC article.
References
- International Telecommunication Union. Market information and statistics. 2007. http://www.itu.int/ITU-D/ict/statistics/maps.html.
- Banjanovic A. Special Report: Towards universal global mobile phone coverage. Euromonitor International; 2009.
- Goodman JW. Introduction to Fourier Optics. Roberts & Company Publishers; Greenwood Village, CO, USA: 2005.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources