Eosinophils in health and disease: the LIAR hypothesis - PubMed (original) (raw)
Eosinophils in health and disease: the LIAR hypothesis
J J Lee et al. Clin Exp Allergy. 2010 Apr.
Abstract
Discussions of eosinophils are often descriptions of end-stage effector cells with destructive capabilities mediated predominantly by released cytotoxic cationic granule proteins. Moreover, eosinophils in the medical literature are invariably associated with the pathologies linked with helminth infections or allergic diseases such as asthma. This has led to an almost fatalist view of eosinophil effector functions and associated therapeutic strategies targeting these cells that would make even William of Ockham proud - eosinophil effector functions have physiological consequences that increase patient morbidity/mortality and 'the only good eosinophils are dead eosinophils'. Unfortunately, the strengths of dogmas are also their greatest weaknesses. Namely, while the repetitive proclamation of dogmatic concepts by authoritative sources (i.e. reviews, meeting proceedings, textbooks, etc.) builds consensus within the medical community and lower the entropies surrounding difficult issues, they often ignore not easily explained details and place diminished importance on alternative hypotheses. The goal of this perspective is twofold: (i) we will review recent observations regarding eosinophils and their activities as well as reinterpret earlier data as part of the synthesis of a new paradigm. In this paradigm, we hypothesize that eosinophils accumulate at unique sites in response to cell turnover or in response to local stem cell activity(ies). We further suggest that this accumulation is part of one or more mechanisms regulating tissue homeostasis. Specifically, instead of immune cells exclusively mediating innate host defence, we suggest that accumulating tissue eosinophils are actually regulators of Local Immunity And/or Remodeling/Repair in both health and disease - the LIAR hypothesis; (ii) we want to be inflammatory (pun intended!) and challenge the currently common perspective of eosinophils as destructive end-stage effector cells. Our hope is to create more questions than we answer and provoke everyone to spend countless hours simply to prove us wrong!
Figures
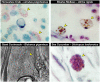
Figure 1. Photomicrographic images of “eosinophil-like” cells from invertebrates representative of three major phyla demonstrates the evolutionary conservation of this granulocyte
Ameboid leukocytes with the distinct granulation and, in many cases, the concomitant eosin-binding characteristics (arrowheads) are found in a wide array of invertebrate species, including Arthropoda (Limulus polyphemus (horseshoe crab - Class: Crustacea, H&E) and Blaberus giganteus (giant cockroach - Class: Insecta, phase contrast microscopy)), Mollusca (Atrina rigida (clam - Class: Bivalvia, H&E), and Echinodermata (Stichopus badionotus (sea cucumber - Class: Holothuroidea, H&E). All photomicrographs were reprinted from
Comparative Hematology
by Warren Andrew (©1965), with permission from Elsevier)
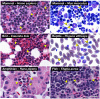
Figure 2. Hematoxylin-eosin (H&E) and Romanowsky-dye (R&D) stained preparations of hematopoietic tissues from representative animals of the five (5) classes of Vertebrata reveal the ubiquitous presence of a uniquely eosinophilic lineage in this sub-phylum
Leukocytes displaying the unique polymorphonucleus and the eosin-binding cytoplasmic granules characteristic of eosinophils are identifiable (arrowheads) in Mammalia (Homo sapiens (human, H&E) and Mus musculus (mouse, R&D)), Aves (Columba livia (rock pigeon, H&E)), Reptilia (Pogona vitticeps (Bearded Dragon, R&D)), Amphibia (Rana pipens (leopard frog, H&E)), and Osteichthyes (Tilapia aurea (Tilapia, H&E)). Scale bar = 20μm.
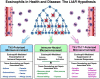
Figure 3. Schematic representation outlining the LIAR hypothesis and the outcomes-based consequences of eosinophil-mediated activities in health and disease
Peripheral eosinophil ( ) recruitment occurs in response to the release of one or more small molecule mediators of inflammation (e.g., DAMPs) released from localized bursts of cell death (
) recruitment occurs in response to the release of one or more small molecule mediators of inflammation (e.g., DAMPs) released from localized bursts of cell death ( ). In the presence of additional eosinophil agonist growth (e.g., IL-5) and survival (e.g., GM-CSF) factors derived from concomitant cell proliferation and/or stem cell activation (
). In the presence of additional eosinophil agonist growth (e.g., IL-5) and survival (e.g., GM-CSF) factors derived from concomitant cell proliferation and/or stem cell activation ( ), these granulocytes accumulate and establish a local steady-state population. The tissue immune microenvironment subsequently dictates the downstream immune consequences mediated by eosinophil effector functions, leading either to exacerbations of local immune responses (Th2-Polarized Microenvironment), suppression of these site-specific immune responses (Th1/Th17-Polarized Microenvironment), or essentially little to no modulations of local immune responses (Immune-Neutral Microenvironment). In turn, these immune responses modulate the levels of tissue remodeling and/or tissue repair that is also characteristic of eosinophil-mediated effector functions. Thus, the immune microenvironment present upon eosinophil recruitment is a significant situational cue which drives the predominance of specific eosinophil activities. More importantly, this eosinophil-mediated **L**ocal **I**mmunity **A**nd/or **Remodeling/R**epair defines the functional roles of eosinophils in unique tissue compartments at homeostatic baseline (i.e., health) as well as within tissues associated with specific diseases.
), these granulocytes accumulate and establish a local steady-state population. The tissue immune microenvironment subsequently dictates the downstream immune consequences mediated by eosinophil effector functions, leading either to exacerbations of local immune responses (Th2-Polarized Microenvironment), suppression of these site-specific immune responses (Th1/Th17-Polarized Microenvironment), or essentially little to no modulations of local immune responses (Immune-Neutral Microenvironment). In turn, these immune responses modulate the levels of tissue remodeling and/or tissue repair that is also characteristic of eosinophil-mediated effector functions. Thus, the immune microenvironment present upon eosinophil recruitment is a significant situational cue which drives the predominance of specific eosinophil activities. More importantly, this eosinophil-mediated **L**ocal **I**mmunity **A**nd/or **Remodeling/R**epair defines the functional roles of eosinophils in unique tissue compartments at homeostatic baseline (i.e., health) as well as within tissues associated with specific diseases.
Figure 4. Histopathological assessments of biopsies from human cancers and tumors from mouse models of cancer show that eosinophil infiltration of tumors is often significant and, more importantly, a widely occurring phenomenon
Immunohistochemistry with a unique and specific monoclonal antibody against the abundant eosinophil secondary granule protein, eosinophil peroxidase (EPX-mAb [73]) demonstrated evidence for eosinophil infiltration in multiple human cancers (darkly staining navy/black cells in each photomicrograph with representative examples noted with arrowheads), including
colon tubular adenoma
,
bladder cancer
,
mammary ductal carcinoma
,
pancreatic cancer
, and
glioblastoma
. In addition, staining with a monoclonal antibody specific for another abundant eosinophil secondary granule protein, major basic protein (rat anti-mouse MBP-mAb-14.7.4 [17]) demonstrated the presence of a robust eosinophil tumor infiltrate occurring in a
mouse model of pancreatic cancer
(Pdx1-Cre (x) KRASG12D/+ mice [82]). Scale bar = 100μm.
Similar articles
- Eosinophils: singularly destructive effector cells or purveyors of immunoregulation?
Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Jacobsen EA, et al. J Allergy Clin Immunol. 2007 Jun;119(6):1313-20. doi: 10.1016/j.jaci.2007.03.043. Epub 2007 May 3. J Allergy Clin Immunol. 2007. PMID: 17481717 Review. - Eosinophils: role in asthma, allergy and parasite immunity.
Kay AB. Kay AB. N Engl Reg Allergy Proc. 1985 Fall;6(4):341-5. doi: 10.2500/108854185779109098. N Engl Reg Allergy Proc. 1985. PMID: 3870502 - Changing roles of eosinophils in health and disease.
Furuta GT, Atkins FD, Lee NA, Lee JJ. Furuta GT, et al. Ann Allergy Asthma Immunol. 2014 Jul;113(1):3-8. doi: 10.1016/j.anai.2014.04.002. Epub 2014 May 1. Ann Allergy Asthma Immunol. 2014. PMID: 24795292 Free PMC article. Review. - Eosinophil granule proteins: form and function.
Acharya KR, Ackerman SJ. Acharya KR, et al. J Biol Chem. 2014 Jun 20;289(25):17406-15. doi: 10.1074/jbc.R113.546218. Epub 2014 May 6. J Biol Chem. 2014. PMID: 24802755 Free PMC article. Review. - A new paradigm of eosinophil granulocytes: neuroimmune interactions.
Raap U, Wardlaw AJ. Raap U, et al. Exp Dermatol. 2008 Sep;17(9):731-8. doi: 10.1111/j.1600-0625.2008.00741.x. Epub 2008 May 26. Exp Dermatol. 2008. PMID: 18505411 Review.
Cited by
- Eosinopenia is associated with greater severity in patients with coronavirus disease 2019.
Zhao L, Zhang YP, Yang X, Liu X. Zhao L, et al. Allergy. 2021 Feb;76(2):562-564. doi: 10.1111/all.14455. Epub 2020 Jul 13. Allergy. 2021. PMID: 32544252 Free PMC article. No abstract available. - Emerging Role of Phospholipase-Derived Cleavage Products in Regulating Eosinophil Activity: Focus on Lysophospholipids, Polyunsaturated Fatty Acids and Eicosanoids.
Knuplez E, Sturm EM, Marsche G. Knuplez E, et al. Int J Mol Sci. 2021 Apr 21;22(9):4356. doi: 10.3390/ijms22094356. Int J Mol Sci. 2021. PMID: 33919453 Free PMC article. Review. - Novel CLC3 transcript variants in blood eosinophils and increased CLC3 expression in nasal lavage and blood eosinophils of asthmatics.
Gaurav R, Bewtra AK, Agrawal DK. Gaurav R, et al. Immun Inflamm Dis. 2014 Dec;2(4):205-13. doi: 10.1002/iid3.36. Epub 2014 Dec 4. Immun Inflamm Dis. 2014. PMID: 25866628 Free PMC article. - Trans-ancestry analysis in over 799,000 individuals yields new insights into the genetic etiology of colorectal cancer.
Yang C, Chang Z, Dai Y, Mo J, Zhang Q, Zhu M, Luan L, Zhang J, Sun B, Jia J. Yang C, et al. PLoS One. 2024 Jul 18;19(7):e0301811. doi: 10.1371/journal.pone.0301811. eCollection 2024. PLoS One. 2024. PMID: 39024248 Free PMC article. - Eosinophils as potential biomarkers in respiratory viral infections.
Macchia I, La Sorsa V, Urbani F, Moretti S, Antonucci C, Afferni C, Schiavoni G. Macchia I, et al. Front Immunol. 2023 Jul 6;14:1170035. doi: 10.3389/fimmu.2023.1170035. eCollection 2023. Front Immunol. 2023. PMID: 37483591 Free PMC article. Review.
References
- Austen KF. Homeostasis of effector systems which can also be recruited for immunologic reactions. J Immunol. 1978;121:793–805. Review. - PubMed
- Smith H, Cook RM. Immunopharmacology of Eosinophils. In: Page CF, editor. The Handbook of Immunopharmacology. 1st ed. Academic Press, Harcourt Brace Jovanovich; London: 1993. p. 250.
- Taliaferro WH, Sarles MP. The cellular reactions in the skin, lungs, and intestine of normal and immune rats after infection with Nippostrongylus muris. J Infect Dis. 1939;64:157–192.
- Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. - PubMed
- Bruschi F, Korenaga M, Watanabe N. Eosinophils and Trichinella infection: toxic for the parasite and the host? Trends Parasitol. 2008;24:462–467. - PubMed
MeSH terms
Grants and funding
- R01 HL078860/HL/NHLBI NIH HHS/United States
- K26 RR019709/RR/NCRR NIH HHS/United States
- R01 HL058723/HL/NHLBI NIH HHS/United States
- R01 HL065228/HL/NHLBI NIH HHS/United States
- R01 CA112442/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources