A formal analysis of cytokine networks in chronic fatigue syndrome - PubMed (original) (raw)
A formal analysis of cytokine networks in chronic fatigue syndrome
Gordon Broderick et al. Brain Behav Immun. 2010 Oct.
Abstract
Chronic Fatigue Syndrome (CFS) is a complex illness affecting 4 million Americans for which no characteristic lesion has been identified. Instead of searching for a deficiency in any single marker, we propose that CFS is associated with a profound imbalance in the regulation of immune function forcing a departure from standard pre-programmed responses. To identify these imbalances we apply network analysis to the co-expression of 16 cytokines in CFS subjects and healthy controls. Concentrations of IL-1a, 1b, 2, 4, 5, 6, 8, 10, 12, 13, 15, 17 and 23, IFN-γ, lymphotoxin-α (LT-α) and TNF-α were measured in the plasma of 40 female CFS and 59 case-matched controls. Cytokine co-expression networks were constructed from the pair-wise mutual information (MI) patterns found within each subject group. These networks differed in topology significantly more than expected by chance with the CFS network being more hub-like in design. Analysis of local modularity isolated statistically distinct cytokine communities recognizable as pre-programmed immune functional components. These showed highly attenuated Th1 and Th17 immune responses in CFS. High Th2 marker expression but weak interaction patterns pointed to an established Th2 inflammatory milieu. Similarly, altered associations in CFS provided indirect evidence of diminished NK cell responsiveness to IL-12 and LT-α stimulus. These observations are consistent with several processes active in latent viral infection and would not have been uncovered by assessing marker expression alone. Furthermore this analysis identifies key sub-networks such as IL-2:IFN-γ:TNF-α that might be targeted in restoring normal immune function.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Fig. 1
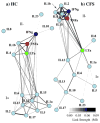
Networks for HC and CFS have visibly different topologies. A weighted spring-electrical embedding structurally reveals the subject-subject (inset) and cytokine-cytokine associations based on measurements in 59 healthy control subjects (a) and 40 CFS patients (b). All edge weights are significant at p ≤ 0.01. Separation of subjects was consistent with their assignment to diagnostic groups supporting the use of within-group variation in the estimation of mutual information for cytokine-cytokine associations.
Fig. 2
Most cytokines significantly modified their connectivity in the CFS state. Theses network alterations were revealed by the relative change in the total weight of edges connected at each node (node degree centrality) as well as edges acquired through first neighbors (normalized eigenvector centrality). Interleukins (IL), 2, 4, and 1b, interferon-gamma (IFNγ), and tumor necrosis factor-alpha (TNFα) became much better integrated into the core network in CFS, while interleukins, 5, 6, 12, 13, and 17 became more weakly associated.
Fig. 3
Both HC and CFS networks are composed of two distinct communities. Visually “relaxing” the links between identified communities of nodes and allowing them to drift apart emphasizes community structure in both networks. Overall modularity was maximized when each network was separated into two communities with differing compositions, labeled I+ at the top and II−. Each community represents a clustering of nodes with a greater internal linkage than would be expected compared to a random sampling of similar nodes.
Comment in
- Letter to the editor re: "A formal analysis of cytokine networks in chronic fatigue syndrome" by Broderick et al.
Carlo-Stella N. Carlo-Stella N. Brain Behav Immun. 2010 Oct;24(7):1218; author reply 1219. doi: 10.1016/j.bbi.2010.06.004. Epub 2010 Jun 16. Brain Behav Immun. 2010. PMID: 20561580 No abstract available.
Similar articles
- Plasma cytokines in women with chronic fatigue syndrome.
Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Fletcher MA, et al. J Transl Med. 2009 Nov 12;7:96. doi: 10.1186/1479-5876-7-96. J Transl Med. 2009. PMID: 19909538 Free PMC article. - Longitudinal analysis of pro- and anti-inflammatory cytokine production in severely fatigued adolescents.
ter Wolbeek M, van Doornen LJ, Kavelaars A, van de Putte EM, Schedlowski M, Heijnen CJ. ter Wolbeek M, et al. Brain Behav Immun. 2007 Nov;21(8):1063-74. doi: 10.1016/j.bbi.2007.04.007. Epub 2007 Jun 1. Brain Behav Immun. 2007. PMID: 17544255 - Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome.
Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schürmeyer TH, Ehlert U. Gaab J, et al. Psychoneuroendocrinology. 2005 Feb;30(2):188-98. doi: 10.1016/j.psyneuen.2004.06.008. Psychoneuroendocrinology. 2005. PMID: 15471616 Clinical Trial. - Cytokines and chronic fatigue syndrome.
Patarca R. Patarca R. Ann N Y Acad Sci. 2001 Mar;933:185-200. doi: 10.1111/j.1749-6632.2001.tb05824.x. Ann N Y Acad Sci. 2001. PMID: 12000020 Review. - Immunological aspects of chronic fatigue syndrome.
Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G. Lorusso L, et al. Autoimmun Rev. 2009 Feb;8(4):287-91. doi: 10.1016/j.autrev.2008.08.003. Epub 2008 Sep 16. Autoimmun Rev. 2009. PMID: 18801465 Review.
Cited by
- Epidemiological and clinical factors associated with post-exertional malaise severity in patients with myalgic encephalomyelitis/chronic fatigue syndrome.
Ghali A, Richa P, Lacout C, Gury A, Beucher AB, Homedan C, Lavigne C, Urbanski G. Ghali A, et al. J Transl Med. 2020 Jun 22;18(1):246. doi: 10.1186/s12967-020-02419-4. J Transl Med. 2020. PMID: 32571354 Free PMC article. - Myalgic encephalomyelitis: International Consensus Criteria.
Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R, Bateman L, Baumgarten-Austrheim B, Bell DS, Carlo-Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall-Gradisnik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Carruthers BM, et al. J Intern Med. 2011 Oct;270(4):327-38. doi: 10.1111/j.1365-2796.2011.02428.x. Epub 2011 Aug 22. J Intern Med. 2011. PMID: 21777306 Free PMC article. Review. - Higher Prevalence of "Low T3 Syndrome" in Patients With Chronic Fatigue Syndrome: A Case-Control Study.
Ruiz-Núñez B, Tarasse R, Vogelaar EF, Janneke Dijck-Brouwer DA, Muskiet FAJ. Ruiz-Núñez B, et al. Front Endocrinol (Lausanne). 2018 Mar 20;9:97. doi: 10.3389/fendo.2018.00097. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29615976 Free PMC article. - Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women.
Milrad SF, Hall DL, Jutagir DR, Lattie EG, Ironson GH, Wohlgemuth W, Nunez MV, Garcia L, Czaja SJ, Perdomo DM, Fletcher MA, Klimas N, Antoni MH. Milrad SF, et al. J Neuroimmunol. 2017 Feb 15;303:43-50. doi: 10.1016/j.jneuroim.2016.12.008. Epub 2016 Dec 14. J Neuroimmunol. 2017. PMID: 28038892 Free PMC article. - Depression, evening salivary cortisol and inflammation in chronic fatigue syndrome: A psychoneuroendocrinological structural regression model.
Milrad SF, Hall DL, Jutagir DR, Lattie EG, Czaja SJ, Perdomo DM, Fletcher MA, Klimas N, Antoni MH. Milrad SF, et al. Int J Psychophysiol. 2018 Sep;131:124-130. doi: 10.1016/j.ijpsycho.2017.09.009. Epub 2017 Sep 14. Int J Psychophysiol. 2018. PMID: 28918107 Free PMC article.
References
- Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318(6047):665–667. - PubMed
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4Tcell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. - PubMed
- Airoldi I, Guglielmino R, Carra G, Corcione A, Gerosa F, Taborelli G, Trinchieri G, Pistoia V. The interleukin-12 and interleukin-12 receptor system in normal and transformed human B lymphocytes. Haematologica. 2002;87(4):434–442. Review. - PubMed
- Barabási AL. Network medicine - from obesity to the “diseasome”. N Engl J Med. 2007;357(4):404–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI065723/AI/NIAID NIH HHS/United States
- R21 AA016636/AA/NIAAA NIH HHS/United States
- R21AA016635/AA/NIAAA NIH HHS/United States
- R01AI065723/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical