FCHo proteins are nucleators of clathrin-mediated endocytosis - PubMed (original) (raw)
FCHo proteins are nucleators of clathrin-mediated endocytosis
William Mike Henne et al. Science. 2010.
Abstract
Clathrin-mediated endocytosis, the major pathway for ligand internalization into eukaryotic cells, is thought to be initiated by the clustering of clathrin and adaptors around receptors destined for internalization. However, here we report that the membrane-sculpting F-BAR domain-containing Fer/Cip4 homology domain-only proteins 1 and 2 (FCHo1/2) were required for plasma membrane clathrin-coated vesicle (CCV) budding and marked sites of CCV formation. Changes in FCHo1/2 expression levels correlated directly with numbers of CCV budding events, ligand endocytosis, and synaptic vesicle marker recycling. FCHo1/2 proteins bound specifically to the plasma membrane and recruited the scaffold proteins eps15 and intersectin, which in turn engaged the adaptor complex AP2. The FCHo F-BAR membrane-bending activity was required, leading to the proposal that FCHo1/2 sculpt the initial bud site and recruit the clathrin machinery for CCV formation.
Figures
Fig. 1
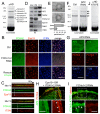
FCHo1/2 proteins are clathrin/AP2 nucleators. (A) Dynamic cell surface localization (top) and kymograph (bottom) of representative CCPs labelled with RFP-FCHo2 (FCHo2) and GFP-LCa (clathrin). FCHo2 was detected before clathrin (white arrows and graph). a.u.: arbitary units. (B) Cryoimmuno-electron microscopy localized GFP-FCHo2 at CCPs. Scale bar 100nm. (C) CCVs, labelled by σ2-GFP (AP2), did not form with double RNAi of FCHo1+2 (FCHo1+2 RNAi) where AP2 became cytosolic (contrast enhanced to show the diffuse signal at the plasma membrane). Inhibition was relieved by co-expression of RNAi-resistant RFP-FCHo2 (rescue). (D) Nucleation rates (number of new CCPs/104μm2/second) in cells treated with scrambled RNAi (Ctrl), RNAi against FCHo1 (1), FCHo2 (2), FCHo1+2 (1+2) or rescue (R). (E) Clathrin ligands, transferrin (Tf) epidermal growth factor (EGF) and low-density lipoprotein (LDL) uptake in cells treated as in C. (F) Clathrin vesicles (AP2) in BSC1 cells transfected with 0, 1 or 2μg of untagged-FCHo2 for 2×105 cells. (G) Nucleation rate and (H) Tf uptake in cells treated as in F. Scale bars, 5μm (C,F) and 200nm (B). Displayed kymographs were representative (percentage, n=319 CCPs (11)).
Fig. 2
FCHo2 directly binds and recruits eps15 and intersectin to initiate CCP maturation. (A) Pull down with GST-FCHo2-μHD and rat brain lysate. Interacting proteins were identified by mass spectrometry. (B) Eps15 and intersectin (ITSN) formed puncta at the PM colocalizing with FCHo2. In double FCHo1+2 RNAi cells, Eps15 and ITSN were cytosolic (contrast enhanced to show the diffuse signal); co-expression of RNAi-resistant FCHo2 (FCHo2r) rescued PM-targeting (Rescue). (C) Kymograph of representative CCPs (percentage, (11)) labeled with FCHo2 and Eps15 or ITSN. (D) FCHo2, eps15, and ITSN were CCV de-enriched. Clathrin (CHC), AP2, and vesicle marker synaptophysin (Syphy) displayed enrichment in CCV fractions (CCV). FCHo2 and ITSN were PM enriched. IB: immunoblot. (E) Cryoimmuno-electron microscopy of GFP-FCHo2 localized it to the CCP neck. Bar graph shows gold particle density in the upper and lower half of constricted CCPs (p<0.01). (F) Pull downs with GST-μHD from scrambled (left) or AP2 RNAi (right) treated HeLa cells. Eps15 and ITSN bands were visible in both (arrows). (G) Upon AP2 depletion (μ2 RNAi) σ2-GFP (another AP2 subunit) was cytosolic (contrast enhanced to show the diffuse signal), but Eps15 and ITSN still co-localize with FCHo2 at the PM (arrows). (H) FCHo2 and AP2 (σ2-GFP) puncta disappeared under Eps15 + Eps15R + ITSN1 + ITSN2 quadruple RNAi. AP2 became cytosolic (diffuse signal) whereas FCHo2 remained at the PM (inset). (I) In FCHo1+2 RNAi cells, RNAi-resistant FCHo2-K797E (FCHo2r-K797E) bound to the PM (arrows) but did not cluster nor rescue CCP formation, reported by RFP-LCa (clathrin). In these cells FCHo1+2 RNAi inhibition of Tf uptake was also not rescued (7.2±3.5% of control uptake (p<0.0001). (J) GST-μHD K797E no longer pulled-down the protein bands visible in A. Green, red and blue panels indicate GFP-, RFP-, and BFP-tagged proteins respectively. Scale bars, 5μm. (B,H,G,I) and 100nm (E).
Fig. 3
Lipid binding and membrane sculpting FCHo1/2 abilities are both essential for CCP formation. (A) Chimeric GFP-PLC-PH+FCHo2μHD (PH-μHD), a dimer interface mutant GFP-FCHo2(F38E+W73E), and RFP-SGIP1 all could not rescue CCP nucleation - monitored by following either clathrin or AP2 fluorescence - in double RNAi FCHo1+2 cells. (B) Lipid co-sedimentation assay of 15μM F-BAR-x in presence of 1mg/mL liposomes: Folch (Avanti brain lipid), FolchPS (80% Folch, 20% phosphatidylserine) or FolchPS +5% of indicated PiPs. Liposome-bound proteins were pelleted (P) by ultracentrifugation, unbound protein remained in the supernatant (S). (C) Folch+15%PS+5%Pi(4,5)P2 liposomes incubated with either 10 or 20μM F-BAR-x and spotted onto EM grids gave mainly tubules of diameters of 50-80nm (10 μM) or 18nm (20μM, most were visibly twisted). Insets show enlargements with protein density striations. (D) F-BAR-x-induced in vivo tubulation: representative images of ‘tubules’ and ‘no tubules’. (E) RNAi-resistant form of full-length FCHo2 (FCHo2r) with membrane-binding mutation (K146E+K163E) remained cyosolic and could not rescue FCHo1/2 RNAi-mediated absence of CCPs, whereas FCHo2r containing membrane-sculpting mutations (I268N and L136E) displayed slowed and aberrant CCPs. Displayed kymographs were representative (percentage, (11)). Table summarizing the mutants and their phenotypes. Scale bars, 5μm (A,D,E) and 100nm (C).
Comment in
- Endocytosis: Curvature proteins direct traffic.
Skinner M. Skinner M. Nat Rev Mol Cell Biol. 2010 Jul;11(7):466. doi: 10.1038/nrm2931. Nat Rev Mol Cell Biol. 2010. PMID: 20571585 No abstract available.
Similar articles
- Eps15 membrane-binding and -bending activity acts redundantly with Fcho1 during clathrin-mediated endocytosis.
Wang L, Johnson A, Hanna M, Audhya A. Wang L, et al. Mol Biol Cell. 2016 Sep 1;27(17):2675-87. doi: 10.1091/mbc.E16-03-0151. Epub 2016 Jul 6. Mol Biol Cell. 2016. PMID: 27385343 Free PMC article. - The clathrin adaptor Dab2 recruits EH domain scaffold proteins to regulate integrin β1 endocytosis.
Teckchandani A, Mulkearns EE, Randolph TW, Toida N, Cooper JA. Teckchandani A, et al. Mol Biol Cell. 2012 Aug;23(15):2905-16. doi: 10.1091/mbc.E11-12-1007. Epub 2012 May 30. Mol Biol Cell. 2012. PMID: 22648170 Free PMC article. - FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis.
Mulkearns EE, Cooper JA. Mulkearns EE, et al. Mol Biol Cell. 2012 Apr;23(7):1330-42. doi: 10.1091/mbc.E11-09-0812. Epub 2012 Feb 9. Mol Biol Cell. 2012. PMID: 22323290 Free PMC article. - Molecular mechanism and physiological functions of clathrin-mediated endocytosis.
McMahon HT, Boucrot E. McMahon HT, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):517-33. doi: 10.1038/nrm3151. Nat Rev Mol Cell Biol. 2011. PMID: 21779028 Review. - Capturing the mechanics of clathrin-mediated endocytosis.
Smith SM, Smith CJ. Smith SM, et al. Curr Opin Struct Biol. 2022 Aug;75:102427. doi: 10.1016/j.sbi.2022.102427. Epub 2022 Jul 21. Curr Opin Struct Biol. 2022. PMID: 35872561 Review.
Cited by
- High-throughput screening of genetic and cellular drivers of syncytium formation induced by the spike protein of SARS-CoV-2.
Chan CWF, Wang B, Nan L, Huang X, Mao T, Chu HY, Luo C, Chu H, Choi GCG, Shum HC, Wong ASL. Chan CWF, et al. Nat Biomed Eng. 2024 Mar;8(3):291-309. doi: 10.1038/s41551-023-01140-z. Epub 2023 Nov 23. Nat Biomed Eng. 2024. PMID: 37996617 Free PMC article. - Molecular mechanisms of contractile-ring constriction and membrane trafficking in cytokinesis.
Gerien KS, Wu JQ. Gerien KS, et al. Biophys Rev. 2018 Dec;10(6):1649-1666. doi: 10.1007/s12551-018-0479-3. Epub 2018 Nov 17. Biophys Rev. 2018. PMID: 30448943 Free PMC article. Review. - Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system.
Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, De Camilli P. Wu M, et al. Nat Cell Biol. 2010 Sep;12(9):902-8. doi: 10.1038/ncb2094. Epub 2010 Aug 22. Nat Cell Biol. 2010. PMID: 20729836 Free PMC article. - Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing.
Zeno WF, Baul U, Snead WT, DeGroot ACM, Wang L, Lafer EM, Thirumalai D, Stachowiak JC. Zeno WF, et al. Nat Commun. 2018 Oct 8;9(1):4152. doi: 10.1038/s41467-018-06532-3. Nat Commun. 2018. PMID: 30297718 Free PMC article. - Role for ERK1/2-dependent activation of FCHSD2 in cancer cell-selective regulation of clathrin-mediated endocytosis.
Xiao GY, Mohanakrishnan A, Schmid SL. Xiao GY, et al. Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):E9570-E9579. doi: 10.1073/pnas.1810209115. Epub 2018 Sep 24. Proc Natl Acad Sci U S A. 2018. PMID: 30249660 Free PMC article.
References
- Traub LM. Nat Rev Mol Cell Biol. 2009;10:583. - PubMed
- Schmid EM, McMahon HT. Nature. 2007;448:883. - PubMed
- Peter BJ, et al. Science. 2004;303:495. - PubMed
- Lundmark R, Carlsson SR. J Biol Chem. 2003;278:46772. - PubMed
- Henne WM, et al. Structure. 2007;15:839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous