Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices - PubMed (original) (raw)
Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices
Yukitoshi Izumi et al. Neurosci Lett. 2010.
Abstract
The activation of N-methyl-D-aspartate (NMDA) receptors and subsequent release of nitric oxide (NO) are likely contributors to the delayed neuronal damage that accompanies ischemia and other neurodegenerative conditions. NMDA receptor antagonists and inhibitors of NO synthesis, however, are of limited benefit when administered following excitotoxic events, suggesting the importance of determining downstream events that result in neuronal degeneration. Inhibition of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), a key glycolytic enzyme, which may result in glycolytic impairment, is one of the biological targets of NO. This suggests that alternative energy substrates may prevent neuronal damage. Using rat hippocampal slices from juvenile rats, we examined the role of glycolytic impairment in NMDA-mediated excitotoxicity and whether pyruvate, an end product of glycolysis, prevents the excitotoxic neuronal injury. We observed that administration of NMDA acutely depresses ATP levels and result in a slowly developing inhibition of GAPDH. Unlike NMDA receptor antagonists or NO inhibitors, exogenously applied pyruvate is effective in restoring ATP levels and preventing delayed neuronal degeneration and synaptic deterioration when administered in the period following NMDA receptor activation. This raises the possibility that treatment with agents that maintain cellular energy function can prevent delayed excitotoxicity.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Figures
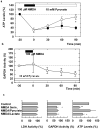
Figure 1
Pyruvate overcomes the depression of ATP and GAPDH by NMDA. a) NMDA (100 μM, filled bar) promptly and persistently depresses ATP levels following a 20 min exposure (filled circles). Administration of 10 mM pyruvate (open bar) immediately after NMDA exposure restores ATP levels (open circles). b) NMDA causes a slow inhibition of GAPDH activity in hippocampal slices. Twenty min exposure to 100 μM NMDA (filled bar) slowly depresses GAPDH activity in the presence of 10 mM pyruvate. Values are ratios relative to pyruvate alone in each experiment. Three or more slices were done at each time point. c) LDH and GAPDH activities and ATP levels were determined 90 min after 5 min exposure to NMDA in 8 hippocampi. ATP level were restored by the presence of 10 mM pyruvate but not by 10 mM L-lactate. Results are normalized with respect to untreated controls. **<0.01, *<0.05 vs. control by paired _t_-test.
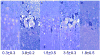
Figure 2
Pyruvate attenuates NMDA-mediated damage in the CA1 region. The photomicrographs depict a) the control appearance of the CA1 region after incubation in standard solution for 110 min; b) the pattern of damage induced by 20 min exposure to 100 μM NMDA followed by 90 min post-incubation in drug free solution; c) preservation of morphological integrity by 10 mM pyruvate administered during NMDA exposure and continuously during the post-incubation period; d) administration of pyruvate only during NMDA exposure; and e) pyruvate administered only in the period after NMDA exposure. NMDA mediated damage in the CA1 region is typically characterized by marked changes in the pyramidal cell layer with severely swollen (pale) neurons interspersed with shrunken (dark) neurons and an overall torn appearance. Numbers below panels depict each damage score. Magnification 275×.
Figure 3
Pyruvate overcomes the depression of EPSPs by NMDA. The graph shows the effects of a 5 min perfusion of 100 μM NMDA (filled bar) on dendritic EPSP slopes in control slices (filled circles, N=5) and in slices treated with 10 mM pyruvate in the period following NMDA (open circles, N=5), or just during the period of NMDA administration (triangles, N=3). Traces were sampled before and 90 min after NMDA administration in the presence of pyruvate or L-lactate.
Similar articles
- Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors.
Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, Pauly JR, Mulholland PJ, Prendergast MA. Butler TR, et al. Neuroscience. 2010 Jan 20;165(2):525-34. doi: 10.1016/j.neuroscience.2009.10.018. Neuroscience. 2010. PMID: 19837138 Free PMC article. - Activation of synaptic NMDA receptors by action potential-dependent release of transmitter during hypoxia impairs recovery of synaptic transmission on reoxygenation.
Sebastião AM, de Mendonca A, Moreira T, Ribeiro JA. Sebastião AM, et al. J Neurosci. 2001 Nov 1;21(21):8564-71. doi: 10.1523/JNEUROSCI.21-21-08564.2001. J Neurosci. 2001. PMID: 11606644 Free PMC article. - 3-Nitropropionic acid toxicity in hippocampus: protection through N-methyl-D-aspartate receptor antagonism.
Karanian DA, Baude AS, Brown QB, Parsons CG, Bahr BA. Karanian DA, et al. Hippocampus. 2006;16(10):834-42. doi: 10.1002/hipo.20214. Hippocampus. 2006. PMID: 16897723 - Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses?
Beal MF. Beal MF. Ann Neurol. 1992 Feb;31(2):119-30. doi: 10.1002/ana.410310202. Ann Neurol. 1992. PMID: 1349466 Review. - Coupling of the NMDA receptor to neuroprotective and neurodestructive events.
Hardingham GE. Hardingham GE. Biochem Soc Trans. 2009 Dec;37(Pt 6):1147-60. doi: 10.1042/BST0371147. Biochem Soc Trans. 2009. PMID: 19909238 Free PMC article. Review.
Cited by
- Robust neuroprotective effects of 2-((2-oxopropanoyl)oxy)-4-(trifluoromethyl)benzoic acid (OPTBA), a HTB/pyruvate ester, in the postischemic rat brain.
Kim SW, Lee HK, Kim ID, Lee H, Luo L, Park JY, Yoon SH, Lee JK. Kim SW, et al. Sci Rep. 2016 Aug 22;6:31843. doi: 10.1038/srep31843. Sci Rep. 2016. PMID: 27545301 Free PMC article. - Pyruvate treatment attenuates cerebral metabolic depression and neuronal loss after experimental traumatic brain injury.
Moro N, Ghavim SS, Harris NG, Hovda DA, Sutton RL. Moro N, et al. Brain Res. 2016 Jul 1;1642:270-277. doi: 10.1016/j.brainres.2016.04.005. Epub 2016 Apr 6. Brain Res. 2016. PMID: 27059390 Free PMC article. - Metabolic Dysfunctions in Amyotrophic Lateral Sclerosis Pathogenesis and Potential Metabolic Treatments.
Tefera TW, Borges K. Tefera TW, et al. Front Neurosci. 2017 Jan 10;10:611. doi: 10.3389/fnins.2016.00611. eCollection 2016. Front Neurosci. 2017. PMID: 28119559 Free PMC article. Review. - Energy substrates protect hippocampus against endogenous glutamate-mediated neurodegeneration in awake rats.
Netzahualcoyotzi C, Tapia R. Netzahualcoyotzi C, et al. Neurochem Res. 2014 Jul;39(7):1346-54. doi: 10.1007/s11064-014-1318-y. Epub 2014 May 1. Neurochem Res. 2014. PMID: 24789366 - The mitochondrial pyruvate carrier at the crossroads of intermediary metabolism.
Yiew NKH, Finck BN. Yiew NKH, et al. Am J Physiol Endocrinol Metab. 2022 Jul 1;323(1):E33-E52. doi: 10.1152/ajpendo.00074.2022. Epub 2022 May 30. Am J Physiol Endocrinol Metab. 2022. PMID: 35635330 Free PMC article. Review.
References
- Dezsi L, Greenberg JH, Sladky J, Araki N, Hamar J, Reivich M. Prolonged effects of MK-801 in the cat during focal cerebral ischemia and recovery: survival, EEG activity and histopathology. J Neurol Sci. 1994;121:110–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- NS57105/NS/NINDS NIH HHS/United States
- MH077791/MH/NIMH NIH HHS/United States
- R01 MH077791/MH/NIMH NIH HHS/United States
- AA017413/AA/NIAAA NIH HHS/United States
- R01 AA017413/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials