Stem cells in the human breast - PubMed (original) (raw)
Review
Stem cells in the human breast
Ole William Petersen et al. Cold Spring Harb Perspect Biol. 2010 May.
Abstract
The origins of the epithelial cells participating in the development, tissue homeostasis, and cancer of the human breast are poorly understood. However, emerging evidence suggests a role for adult tissue-specific stem cells in these processes. In a hierarchical manner, these generate the two main mammary cell lineages, producing an increasing number of cells with distinct properties. Understanding the biological characteristics of human breast stem cells and their progeny is crucial in attempts to compare the features of normal stem cells and cancer precursor cells and distinguish these from nonprecursor cells and cells from the bulk of a tumor. A historical overview of research on human breast stem cells in primary tissue and in culture reveals the progress that has been made in this area, whereas a focus on the cell-of-origin and reprogramming that occurs during neoplastic conversion provides insight into the enigmatic way in which human breast cancers are skewed toward the luminal epithelial lineage.
Figures
Figure 1.
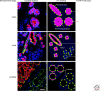
Fetal human breast. Schematic representation of a fetal human breast at the primary bud stage (upper row), the secondary bud stage (middle row), and the neonatal stage (lower row). Nuclei, keratin K14 and keratin K19 are represented by blue, green, and red, respectively. At the primary bud stage, the human breast primordium consists of an inner layer of central primary bud cells and an outer layer of basal primary bud cells surrounded by mesenchyme. Significantly, the basal primary bud cells differ from the basal epidermal cells by the lack of keratin K14. At the secondary bud stage mammary projections are characterized by the dual expression of keratins K14 and K19. At the neonatal stage the luminal and basal epithelial lineages are clearly separated. Double-positive cells for keratin K14 and K19 are scattered in terminal lobular units and end buds.
Figure 2.
Postnatal human breast. Multicolor imaging (left column) and schematic representation (right column) of cryostat sections of the human breast at the infant (upper row), adult (middle row), and lactating (lower row) stage stained against nuclei (blue), keratin K14 (green), and keratin K19 (red). Whereas the infant breast consists of up to three cell types in terms of keratin K14 and K19 expression (−/+; +/−, and rare +/+) the adult breast shows a fourth cell type being double-negative (−/−). These cells are luminal and may expand clonally and differentiate during lactation side by side with +/− lobules (Scale bar = 50 µm).
Figure 3.
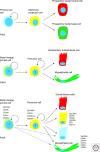
Schematic model of the human breast lineage hierarchy at different developmental stages. The most primitive fetal cells are double-negative for keratin K14 and K19 represented with a blue cytoplasm. Stem cells in the infant and adult human breast are thought to be lineage primed along the basal lineage represented by a light green cytoplasm. Multipotent progenitor cells are double-positive for keratin K14 and K19 represented by a yellow cytoplasm. Differentiated breast basal/myoepithelial and epithelial cells are either positive for keratin K14 (green), keratin K19 (red), double positive (yellow), or double negative (blue). Additional markers are listed for the different classes of cells in the adult breast.
Figure 4.
Keratin K19/K14 breast cancer subtypes. Multicolor imaging of cryostat sections of human breast carcinomas representing different subtypes as defined by combinations of staining with keratin K19 and K14. In a sample of 50 randomly selected primary breast carcinomas, 6 were double-positive for keratin K19 and K14 (A), 43 were positive for keratin K19 only (B), 1 was double-negative (C), and none of them were positive for keratin K14 alone (D; staining of a malignant myoepithelioma from a different sample) (Scale bar = 50 µm).
Comment in
- On stem cells in the human breast.
LaBarge MA. LaBarge MA. Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a013441. doi: 10.1101/cshperspect.a013441. Cold Spring Harb Perspect Biol. 2012. PMID: 22550235 Free PMC article. Review.
Similar articles
- The mammary stem cell hierarchy: a looking glass into heterogeneous breast cancer landscapes.
Sreekumar A, Roarty K, Rosen JM. Sreekumar A, et al. Endocr Relat Cancer. 2015 Dec;22(6):T161-76. doi: 10.1530/ERC-15-0263. Epub 2015 Jul 23. Endocr Relat Cancer. 2015. PMID: 26206777 Free PMC article. Review. - Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties.
Gudjonsson T, Villadsen R, Nielsen HL, Rønnov-Jessen L, Bissell MJ, Petersen OW. Gudjonsson T, et al. Genes Dev. 2002 Mar 15;16(6):693-706. doi: 10.1101/gad.952602. Genes Dev. 2002. PMID: 11914275 Free PMC article. - In search of a stem cell hierarchy in the human breast and its relevance to breast cancer evolution.
Villadsen R. Villadsen R. APMIS. 2005 Nov-Dec;113(11-12):903-21. doi: 10.1111/j.1600-0463.2005.apm_344.x. APMIS. 2005. PMID: 16480457 Review. - [Recent advances on study of human breast stem cells].
Chen DB, Shen DH, Kan X. Chen DB, et al. Zhonghua Bing Li Xue Za Zhi. 2007 Jun;36(6):423-5. Zhonghua Bing Li Xue Za Zhi. 2007. PMID: 17822633 Review. Chinese. No abstract available. - Mammary gland stem cells: current status and future challenges.
Fridriksdottir AJ, Petersen OW, Rønnov-Jessen L. Fridriksdottir AJ, et al. Int J Dev Biol. 2011;55(7-9):719-29. doi: 10.1387/ijdb.113373af. Int J Dev Biol. 2011. PMID: 22161829 Review.
Cited by
- The mammary stem cell hierarchy: a looking glass into heterogeneous breast cancer landscapes.
Sreekumar A, Roarty K, Rosen JM. Sreekumar A, et al. Endocr Relat Cancer. 2015 Dec;22(6):T161-76. doi: 10.1530/ERC-15-0263. Epub 2015 Jul 23. Endocr Relat Cancer. 2015. PMID: 26206777 Free PMC article. Review. - Cancer cell of origin: spotlight on luminal progenitors.
Chaffer CL, Weinberg RA. Chaffer CL, et al. Cell Stem Cell. 2010 Sep 3;7(3):271-2. doi: 10.1016/j.stem.2010.08.008. Cell Stem Cell. 2010. PMID: 20804960 Free PMC article. - Intermediate Filaments as Effectors of Cancer Development and Metastasis: A Focus on Keratins, Vimentin, and Nestin.
Sharma P, Alsharif S, Fallatah A, Chung BM. Sharma P, et al. Cells. 2019 May 23;8(5):497. doi: 10.3390/cells8050497. Cells. 2019. PMID: 31126068 Free PMC article. Review. - Differential in vivo tumorigenicity of distinct subpopulations from a luminal-like breast cancer xenograft.
Skrbo N, Hjortland GO, Kristian A, Holm R, Nord S, Prasmickaite L, Engebraaten O, Mælandsmo GM, Sørlie T, Andersen K. Skrbo N, et al. PLoS One. 2014 Nov 24;9(11):e113278. doi: 10.1371/journal.pone.0113278. eCollection 2014. PLoS One. 2014. PMID: 25419568 Free PMC article. - Comparison of the transcriptomes of long-term label retaining-cells and control cells microdissected from mammary epithelium: an initial study to characterize potential stem/progenitor cells.
Choudhary RK, Li RW, Evock-Clover CM, Capuco AV. Choudhary RK, et al. Front Oncol. 2013 Feb 15;3:21. doi: 10.3389/fonc.2013.00021. eCollection 2013. Front Oncol. 2013. PMID: 23423481 Free PMC article.
References
- Anbazhagan R, Osin PP, Bartkova J, Nathan B, Lane EB, Gusterson BA 1998. The development of epithelial phenotypes in the human fetal and infant breast. J Pathol 184:197–206 - PubMed
- Bartek J, Bartkova J, Kyprianou N, Lalani E-N, Staskova Z, Shearer M, Chang S, Taylor-Papadimitriou J 1991. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with recombinant retrovirus. Proc Natl Acad Sci 88:3520–3524 - PMC - PubMed
- Bartek J, Taylor-Papadimitriou J, Miller N, Millis R 1985. Patterns of expression of keratin 19 as detected with monoclonal antibodies in human breast tissues and tumours. Int J Cancer 36:299–306 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA064786/CA/NCI NIH HHS/United States
- CA116235/CA/NCI NIH HHS/United States
- P01 CA080111/CA/NCI NIH HHS/United States
- P50 CA089393/CA/NCI NIH HHS/United States
- R01 CA116235/CA/NCI NIH HHS/United States
- CA143233/CA/NCI NIH HHS/United States
- CA080111/CA/NCI NIH HHS/United States
- CA89393/CA/NCI NIH HHS/United States
- U01 CA143233/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical