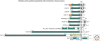Involvement of members of the cadherin superfamily in cancer - PubMed (original) (raw)
Review
Involvement of members of the cadherin superfamily in cancer
Geert Berx et al. Cold Spring Harb Perspect Biol. 2009 Dec.
Abstract
We review the role of cadherins and cadherin-related proteins in human cancer. Cellular and animal models for human cancer are also dealt with whenever appropriate. E-cadherin is the prototype of the large cadherin superfamily and is renowned for its potent malignancy suppressing activity. Different mechanisms for inactivating E-cadherin/CDH1 have been identified in human cancers: inherited and somatic mutations, aberrant protein processing, increased promoter methylation, and induction of transcriptional repressors such as Snail and ZEB family members. The latter induce epithelial mesenchymal transition, which is also associated with induction of "mesenchymal" cadherins, a hallmark of tumor progression. VE-cadherin/CDH5 plays a role in tumor-associated angiogenesis. The atypical T-cadherin/CDH13 is often silenced in cancer cells but up-regulated in tumor vasculature. The review also covers the status of protocadherins and several other cadherin-related molecules in human cancer. Perspectives for emerging cadherin-related anticancer therapies are given.
Figures
Figure 1.
Schematic overview of representative human members of the cadherin superfamily with reported involvement in cancer (modified after Hulpiau and van Roy 2009). All proteins are drawn to scale and aligned at their transmembrane domain (TM). Their total sizes are indicated on the right (number of amino acid residues). The following protein domains are shown: CBD, (conserved cadherin-specific) catenin binding domain; CD, unique cytoplasmic domain; CE, Cysteine-rich EGF repeat-like domain; CM1 to CM3, conserved motifs in the CDs of δ-protocadherins; EC, extracellular cadherin repeat; GPI, glycosylphosphatidylinositol anchor; JMD, (conserved cadherin-specific) juxtamembrane domain; LAG, laminin A globular domain; Pro-d, prodomain; TK, tyrosine kinase domain. On the basis of a phylogenetic analysis (Hulpiau and van Roy 2009), it was proposed that protocadherin LKC (PC-LKC or protocadherin-24) should be renamed (CDHR24).
Figure 2.
Various levels at which E-cadherin expression is regulated in human tumors (modified, with permission, from van Roy and Berx 2008). The E-cadherin gene CDH1 is on chromosome 16q22.1 (depicted at the bottom). This region frequently shows loss of heterozygosity (LOH) in different human carcinoma types. Specific inactivating mutations are scattered throughout the whole coding region and are particularly abundant in sporadic lobular breast cancer and diffuse gastric cancer. Germline mutations can also occur; they cause the hereditary diffuse gastric cancer syndrome. Furthermore, post-translational modifications, such as phosphorylation and glycosylation, and proteolytic processing can affect E-cadherin protein functionality. Epigenetic silencing has been associated with CpG methylation in the CDH1 promoter region or with direct binding of specific transcriptional repressors to E-box sequences in this region. The transcriptional repressors ZEB1/δEF1 and ZEB2/SIP1 are repressed in epithelia by the miRNAs of the miR-200 family. In turn, the ZEB transcription factors down-regulate transcription of the miR-200 genes. Thus, a biphasic regulatory system controls the balance between the epithelial and mesenchymal status in response to incoming signals. TGF-β in the tumor microenvironment can induce the expression of ZEB proteins, at least in part by down-regulating the miR-200 family members. This results in a self-enhancing loop that leads to epithelial dedifferentiation and invasion. See text for more details and references. AA, amino acid position; C, carboxy-terminal end; CD, cytoplasmic domain; EC, extracellular cadherin repeat; N, amino-terminal end; PRO, propeptide; S, signal peptide; TM, transmembrane region. The arrows point to the transcriptional initiation start.
Figure 3.
Schematic overview of the E-cadherin-catenin complex and of δ1-protocadherins at the junction between two adjacent cells (modified, with permission, from Redies et al. 2005; van Roy and Berx 2008). Top: The armadillo catenins p120ctn and β-catenin/plakoglobin bind to, respectively, membrane-proximal and carboxy-terminal halves of the cytoplasmic domain of E-cadherin. This increases junction strength and stability. As extensively described in the literature, both β-catenin and p120ctn also have cancer-related roles in the cytoplasm and in the nucleus. Monomeric α-catenin binds to the E-cadherin cytoplasmic domain via β-catenin, whereas dimeric α-catenin can bind and cross-link filamentous actin (F-actin). Moreover, dimeric EPLIN forms a link between the E-cadherin-catenin complex and F-actin. See text for more details and references. Bottom: The evidence for the depicted δ1-protocadherins structure is circumstantial, but the following features are typical: seven extracellular cadherin repeats (EC) instead of five and a completely different cytoplasmic domain with conserved motifs (CM). The CM3 or RVTF motif has been shown to interact with phosphatase PP1α, probably resulting in its inactivation (reviewed in Redies et al. 2005).
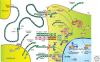
Figure 4.
Selection of expression patterns and activities of members of the cadherin superfamily in cancer. Three cell types are partly depicted: a cancer cell, an endothelial cell, and another type of stromal cell. Protein domains in green are strongly homologous to those in the prototypic E-cadherin/CDH1. Domains in other colors deviate substantially in structure and function. Black dots represent Ca2+ ions. Arrows with minus sign symbols refer to direct or indirect inhibitory influences. Cadherins shown at the cancer cell surface are expressed at the apical membrane (T-cadherin/CDH13 above the tight junction, TJ), or at lateral membranes (E-cadherin/CDH1, N-cadherin/CDH2, cadherin-11/CDH11). The latter three probably occur as _cis_-homodimers. CDH1 is frequently inactivated in cancer cells, whereas the “mesenchymal” cadherins CDH2 and CDH11 are often up-regulated. CDH2 can interact with fibroblast growth factor receptor (FGFR), potentiating its signaling through the enzymes MAPK, PLCγ, and PI3K. Cadherins are prone to proteolytic processing (scissors symbols), which releases either the ectodomain or a carboxy-terminal fragment (CTF). In the case of CDH2, this CTF has been shown to enter the nucleus and inhibit the CREB binding protein (CBP). On neural activation, CDH2 and CDH11 associate with protocadherin-8 (PCDH8 or Arcadlin), which results in activation of the MAPKKK TAO2β, eventually leading to endocytosis of the cadherins. PCDH8 is often silenced in cancer cells, but it is unclear whether this is causally linked to up-regulation of mesenchymal cadherins. The transcriptional activity of β-catenin (β-ctn) in a nuclear complex with LEF/TCF, leads, amongst other effects, to androgen receptor (AR)-independent prostate cancer growth. This phenomenon is inhibited by sequestration of β-ctn by E-cadherin or by degradation of β-ctn after phosphorylation (-P) by GSK3β. Degradation of β-ctn occurs in a cytoplasmic degradation complex with APC (adenomatous polyposis coli protein), Axin, and Disheveled (DSH). However, nuclear β-ctn-LEF/TCF activity is stimulated by an unknown mechanism by a cytoplasmic variant of protocadherin-11Y (PCDH11Y) lacking a signal peptide because of a truncated aminoterminus (ΔN). Tumor-associated endothelial cells express VE-cadherin/CDH5, CDH2, and T-cadherin/CDH13. The latter is linked to the PM via a glycosylphosphatidylinositol (GPI) anchor and signals via secreted Grp78/BiP to an anti-apoptotic PI3K-AKT pathway. Dachsous-1 (DCHS1) and FAT4 are both huge cadherin-related proteins, interacting with each other in heterophilic (different protein types) and heterotypic (different cell types) ways. Silencing of human FAT4 is seen in breast cancer and its activation is linked in an unresolved way to the Hippo-YAP pathway, which controls organ size in Drosophila and is affected in several human cancers. See text for details and references. (a.o.) Amongst others; (EC) extracellular cadherin repeat; (PM) plasma membrane; (TK) tyrosine kinase domain.
Similar articles
- Beyond E-cadherin: roles of other cadherin superfamily members in cancer.
van Roy F. van Roy F. Nat Rev Cancer. 2014 Feb;14(2):121-34. doi: 10.1038/nrc3647. Epub 2014 Jan 20. Nat Rev Cancer. 2014. PMID: 24442140 Review. - Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer.
Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn CO, Heidecke CD, Friess H, Büchler M, Evert M, Lerch MM, Weiss FU. Aghdassi A, et al. Gut. 2012 Mar;61(3):439-48. doi: 10.1136/gutjnl-2011-300060. Epub 2011 Dec 5. Gut. 2012. PMID: 22147512 - Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells.
Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI, Hong SP, Hong SD. Hong KO, et al. J Exp Clin Cancer Res. 2009 Feb 26;28(1):28. doi: 10.1186/1756-9966-28-28. J Exp Clin Cancer Res. 2009. PMID: 19243631 Free PMC article. - Cadherins and epithelial-to-mesenchymal transition.
Gheldof A, Berx G. Gheldof A, et al. Prog Mol Biol Transl Sci. 2013;116:317-36. doi: 10.1016/B978-0-12-394311-8.00014-5. Prog Mol Biol Transl Sci. 2013. PMID: 23481201 Review. - Cadherins: The Superfamily Critically Involved in Breast Cancer.
Ashaie MA, Chowdhury EH. Ashaie MA, et al. Curr Pharm Des. 2016;22(5):616-38. doi: 10.2174/138161282205160127095338. Curr Pharm Des. 2016. PMID: 26825466 Review.
Cited by
- Molecular Mechanisms in Tumorigenesis of Hepatocellular Carcinoma and in Target Treatments-An Overview.
Szilveszter RM, Muntean M, Florea A. Szilveszter RM, et al. Biomolecules. 2024 Jun 4;14(6):656. doi: 10.3390/biom14060656. Biomolecules. 2024. PMID: 38927059 Free PMC article. Review. - Overexpression of wild type or a Q311E mutant MB21D2 promotes a pro-oncogenic phenotype in HNSCC.
Gracilla DE, Korla PK, Lai MT, Chiang AJ, Liou WS, Sheu JJ. Gracilla DE, et al. Mol Oncol. 2020 Dec;14(12):3065-3082. doi: 10.1002/1878-0261.12806. Epub 2020 Oct 15. Mol Oncol. 2020. PMID: 32979859 Free PMC article. - ALCAM regulates motility, invasiveness, and adherens junction formation in uveal melanoma cells.
Jannie KM, Stipp CS, Weiner JA. Jannie KM, et al. PLoS One. 2012;7(6):e39330. doi: 10.1371/journal.pone.0039330. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22745734 Free PMC article. - Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo.
Kaur H, Phillips-Mason PJ, Burden-Gulley SM, Kerstetter-Fogle AE, Basilion JP, Sloan AE, Brady-Kalnay SM. Kaur H, et al. Mol Cancer Res. 2012 Mar;10(3):293-304. doi: 10.1158/1541-7786.MCR-11-0457. Epub 2012 Jan 20. Mol Cancer Res. 2012. PMID: 22267545 Free PMC article. - CDH1 methylation in preoperative peritoneal washes is an independent prognostic factor for gastric cancer.
Yu QM, Wang XB, Luo J, Wang S, Fang XH, Yu JL, Ling ZQ. Yu QM, et al. J Surg Oncol. 2012 Nov;106(6):765-71. doi: 10.1002/jso.23116. Epub 2012 Apr 18. J Surg Oncol. 2012. PMID: 22514028 Free PMC article.
References
- Adachi Y, Takeuchi T, Sonobe H, Ohtsuki Y 2006. An adiponectin receptor, T-cadherin, was selectively expressed in intratumoral capillary endothelial cells in hepatocellular carcinoma: Possible cross talk between T-cadherin and FGF-2 pathways. Virchows Archiv 448:311–318 - PubMed
- Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH 1999. A novel human xenograft model of inflammatory breast cancer. Cancer Res 59:5079–5084 - PubMed
- Anders J, Kjaer S, Ibanez CF 2001. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem 276:35808–35817 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous