Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory - PubMed (original) (raw)
Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory
E Y Yuen et al. Mol Psychiatry. 2011 Feb.
Abstract
Corticosteroid stress hormones have a strong impact on the function of prefrontal cortex (PFC), a central region controlling cognition and emotion, though the underlying mechanisms are elusive. We found that behavioral stressor or short-term corticosterone treatment in vitro induces a delayed and sustained potentiation of the synaptic response and surface expression of N-methyl-D-aspartic acid receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) in PFC pyramidal neurons through a mechanism depending on the induction of serum- and glucocorticoid-inducible kinase (SGK) and the activation of Rab4, which mediates receptor recycling between early endosomes and the plasma membrane. Working memory, a key function relying on glutamatergic transmission in PFC, is enhanced in acutely stressed animals through an SGK-dependent mechanism. These results suggest that acute stress, by activating glucocorticoid receptors, increases the trafficking and function of NMDARs and AMPARs through SGK/Rab4 signaling, which leads to the potentiated synaptic transmission, thereby facilitating cognitive processes mediated by the PFC.
Figures
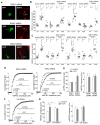
Figure 1. Acute stress or in vitro corticosterone treatment enhances the synaptic response and surface expression of NMDARs and AMPARs in PFC pyramidal neurons via activation of GRs
A–C, Summarized input-output curves of NMDAR-EPSC (A), AMPAR-EPSC (B) or GABAAR-IPSC (C) evoked by a series of stimulus intensities in PFC pyramidal neurons taken from control or animals exposed to forced-swim stress (examined at 1–4 hr, 24 hr and 5 days post-stress). Inset: Representative synaptic current traces. Scale bars: 100 pA, 100 ms (A); 50 pA, 20 ms (B); 50 pA, 40 ms (C). *: p<0.001. D, Dot plot showing the amplitude of NMDAR-EPSC in PFC neurons treated without or with corticosterone (cort, 100 nM, 20 min), RU486 (20 μM, 20 min), RU486+cort, vehicle (DMSO, 20 min), dexamethasone (DEX, 100 nM, 20 min), aldosterone (Aldo, 10 nM, 20 min) or corticotrophin releasing factor (CRF, 200 nM, 20 min). Recordings were obtained 1–4 hr after the treatment. E,F, Plots of NMDAR-EPSC and AMPAR-EPSC amplitude recorded in PFC slices (E) or NMDAR and AMPAR current density recorded in PFC cultures (F) at various time points post-corticosterone treatment (100 nM, 20 min). G, Immunocytochemical images of surface GFP-NR2A (A), GFP-NR2B (B) and GluR1 (C) in PFC cultures treated without (control) or with corticosterone (100 nM, 20 min). Enlarged versions of the boxed regions of dendrites are shown beneath each of the images. H, Quantitative analysis of surface NR2A, NR2B and GluR1 clusters (cluster density, cluster size and cluster intensity) along dendrites in control vs. corticosterone-treated PFC cultures. #: p<0.05, *: p<0.001. I, Immunocytochamical images (upper panel) and quantitative analysis (lower panel) of synaptic GluR1 (PSD-95 co-localized, yellow puncta), total GluR1 clusters (red puncta) and PSD-95 clusters (green puncta) along dendrites in control vs. corticosterone (100 nM, 20 min)-treated PFC cultures. Enlarged versions of the boxed regions of dendrites are also shown. *: p<0.001.
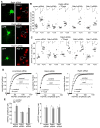
Figure 2. SGK is upregulated in PFC by acute stress via GR and is necessary for acute stress-induced potentiation of glutamatergic transmission
A,B, Western blots (A) and quantification (B) of SGKs in lysates of PFC slices taken from control or stressed animals at various post-stress time points (0 min, 30 min, 1 hr and 2 hr). *: p<0.001. C, Western blots and quantification of SGKs in lysates of PFC slices taken from control or stressed animals without or with i.p. injection of RU486 (10 mg/kg, administered 30 min before stress). Blotting was done 1 hr post-stress. *: p<0.001. D,E, Dot plots of NMDAR-EPSC (D) or AMPAR-EPSC (E) recorded in PFC slices treated with or without corticosterone (100 nM, 20 min) in the presence of TAT-SGK peptide (1μM), scrambled peptide (TAT-sc, 1μM), Akt Inhibitor V (20 μM) or p42/44 MAPK kinase inhibitor PD98059 (40 μM). Peptides or compounds were added 30 min prior to corticosterone. Recordings were performed at 1–4 hrs after corticosterone treatment. F, Dot plots of NMDAR-EPSC recorded in PFC slices from control vs. stressed animals i.v. injected with TAT-SGK peptide (0.6 pmol/g) or a scrambled control peptide (TAT-sc, 0.6 pmol/g). Peptides were administered 30 min prior to stress, and recordings were performed at 1–4 hrs post-stress. Inset (D–F): Representative NMDAR-EPSC or AMPAR-EPSC traces. Scale bars: 100 pA, 100 ms (NMDA); 50 pA, 20 ms (AMPA). G,H, Representative mEPSC traces (G) and bar graphs of mEPSC amplitude (mean ± SEM, H) in PFC slices from control vs. stressed animals i.v. injected with different peptides. Scale bars (G): 10 pA, 1 s. *: p<0.001.
Figure 3. SGK1 and SGK3 are involved in corticosterone enhancement of NMDAR and AMPAR currents
A, Immunocytochemical staining of SGK1, SGK2, or SGK3 in cultured PFC neurons transfected with siRNA against each SGK isoform. GFP was co-transfected to illuminate positive neurons. B,C, Dot plots showing the effect of corticosterone treatment (100 nM, 20 min) on NMDAR (B) or AMPAR (C) current density in PFC cultures transfected with a scrambled siRNA or siRNA against each SGK isoform. The siRNA-resistant silent mutant of SGK1 (RSGK1) or SGK3 (RSGK3) was co-transfected in the rescue experiments. Recordings were obtained 1–4 hr after the treatment. Inset: Representative current traces. Scale bars: 200 pA, 1 sec. D,F, Cumulative distribution of mEPSC amplitude in PFC cultures (DIV21) transfected with SGK1 siRNA or SGK2 siRNA (D), or constitutively activating SGK3 (CA-SGK3, F). Two or three days after transfection, neurons were treated with or without corticosterone (100 nM) for 20 min, and recorded 24 hr later. Inset: Representative mEPSC traces. Scale bar: 50 pA, 1 sec. E,G, Bar graphs (mean ± SEM) showing the mEPSC amplitude and frequency with corticosterone treatment in neurons transfected with different siRNAs (E) or constructs (G). *: p<0.01.
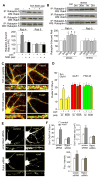
Figure 4. Corticosterone increases NMDAR and AMPAR currents via a Rab4-dependent mechanism
A, Immunocytochemical staining of Rab4, Rab5 or Rab11 in cultured PFC neurons transfected with siRNA against each Rab member. GFP was co-transfected to illuminate positive neurons. B,C, Dot plots showing the effect of corticosterone treatment (100 nM, 20 min) on NMDAR (B) or AMPAR (C) current density in PFC cultures transfected with a scrambled siRNA or siRNA against Rab4, Rab5 or Rab11. The siRNA-resistant silent mutant of Rab4 (RRab4) was co-transfected in the rescue experiments. Recordings were obtained 1–4 hr after the treatment. Inset: Representative current traces. Scale bars: 200 pA, 1 sec. D, Cumulative distribution of mEPSC amplitude in PFC cultures (DIV21) transfected with a scrambled siRNA, Rab4 siRNA, or Rab5 siRNA. Two or three days after transfection, neurons were treated with or without corticosterone (100 nM) for 20 min, and recorded 24 hr later. Inset: Representative mEPSC traces. Scale bar: 50 pA, 1 sec. E, Bar graphs (mean ± SEM) showing the mEPSC amplitude and frequency with corticosterone treatment in neurons transfected with different siRNAs. *: p<0.001.
Figure 5. Corticosterone activates Rab4 through a SGK-dependent mechanism, and SGK inhibition or Rab4 knockdown blocks the enhancing effect of corticosterone on glutamate receptor trafficking
A,B, Top panel: Co-immuoprecipitation blots showing the level of active (Rabaptin-5-bound) Rab4 or Rab5 in PFC slices without or with corticosterone treatment (100 nM, 20 min, collected 1 hr after treatment) in the absence or presence of TAT-SGK peptide (50 μM, 30 min prior to corticosterone, A), or in PFC slices from control vs. swim-stressed animals examined at various post-stress time points (B). Lower panel: Quantification showing the normalized level of Rabaptin-5-bound (active) Rab4 or Rab5 in PFC slices without vs. with corticosterone treatment (A) or from control vs. stressed animals (B). *: p<0.001. C,D, Immunocytochamical images (C) and quantitative analysis (D) of synaptic GluR1 clusters (PSD-95 co-localized, yellow puncta), total GluR1 clusters (red puncta) and PSD-95 clusters (green puncta) in control vs. corticosterone (100 nM, 20 min)-treated PFC pyramidal neurons from cultures pre-treated with TAT-SGK peptide (50 μM) or the scrambled control peptide (TAT-sc). Enlarged versions of the boxed dendritic regions are also shown. *: p<0.001. E, Immunocytochemical images of surface GluR1 in cultured PFC neurons (DIV21–23) transfected with a scrambled siRNA or Rab4 siRNA. Two or three days after transfection, neurons were treated with or without corticosterone (100 nM) for 20 min, and stained 24 hrs later. F, Quantitative analysis of surface GluR1 clusters along dendrites in control vs. corticosterone-treated PFC cultures with different transfections. #: p<0.05, *: p<0.001.
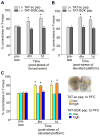
Figure 6. Acute behavioral stress enhances working memory via a SGK-dependent mechanism
A,B, Cumulative data (mean ± SEM) showing the percentage correctness in T-maze tests before and after forced-swim stress (A) or elevated platform stress (B) in rats i.v. injected with TAT-SGK peptide vs. scrambled TAT-sc peptide (0.6 pmol/g). *: p<0.01. C, Cumulative data (mean ± SEM) showing the percentage correctness in T-maze tests before and after elevated platform stress in rats locally injected to PFC with TAT-SGK peptide vs. scrambled TAT-sc peptide (high dose: 40 pmol/g; low dose: 0.12 pmol/g). Inset: A photograph showing the slice with a local injection of ink to PFC prelimbic regions to confirm the appropriate location of the cannula. *: p<0.01.
Similar articles
- Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Yuen EY, et al. Neuron. 2012 Mar 8;73(5):962-77. doi: 10.1016/j.neuron.2011.12.033. Neuron. 2012. PMID: 22405206 Free PMC article. - Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation.
Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Foster KA, et al. J Neurosci. 2010 Feb 17;30(7):2676-85. doi: 10.1523/JNEUROSCI.4022-09.2010. J Neurosci. 2010. PMID: 20164351 Free PMC article. - Molecular and Epigenetic Mechanisms for the Complex Effects of Stress on Synaptic Physiology and Cognitive Functions.
Yuen EY, Wei J, Yan Z. Yuen EY, et al. Int J Neuropsychopharmacol. 2017 Nov 1;20(11):948-955. doi: 10.1093/ijnp/pyx052. Int J Neuropsychopharmacol. 2017. PMID: 29016816 Free PMC article. Review. - Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders.
Gamo NJ, Arnsten AF. Gamo NJ, et al. Behav Neurosci. 2011 Jun;125(3):282-96. doi: 10.1037/a0023165. Behav Neurosci. 2011. PMID: 21480691 Free PMC article. Review.
Cited by
- Glucocorticoids Rapidly Modulate CaV1.2-Mediated Calcium Signals through Kv2.1 Channel Clusters in Hippocampal Neurons.
Wan D, Lu T, Li C, Hu C. Wan D, et al. J Neurosci. 2024 Nov 6;44(45):e0179242024. doi: 10.1523/JNEUROSCI.0179-24.2024. J Neurosci. 2024. PMID: 39299804 - Effects of chronic stress on cognitive function - From neurobiology to intervention.
Girotti M, Bulin SE, Carreno FR. Girotti M, et al. Neurobiol Stress. 2024 Sep 2;33:100670. doi: 10.1016/j.ynstr.2024.100670. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39295772 Free PMC article. Review. - Depression and the Glutamate/GABA-Glutamine Cycle.
Mamelak M. Mamelak M. Curr Neuropharmacol. 2024;23(1):75-84. doi: 10.2174/1570159X22666240815120244. Curr Neuropharmacol. 2024. PMID: 39150032 Review. - The Involvement of the Serotonergic System in Ketamine and Fluoxetine Combination-induced Cognitive Impairments in Mice.
Uyar E, Erdinç M, Kelle İ, Erdinç L, Şeker U, Nergiz Y. Uyar E, et al. Eurasian J Med. 2024 Jun;56(2):102-107. doi: 10.5152/eurasianjmed.2024.23219. Eurasian J Med. 2024. PMID: 39128082 Free PMC article. - Multiplexed Membrane Signaling by Glucocorticoids.
Harrison LM, Tasker JG. Harrison LM, et al. Curr Opin Endocr Metab Res. 2022 Oct;26:100390. doi: 10.1016/j.coemr.2022.100390. Epub 2022 Aug 24. Curr Opin Endocr Metab Res. 2022. PMID: 38075196 Free PMC article.
References
- McEwen BS. Protective and damaging effects of stress mediators. N engl J Med. 1998;338:171–9. - PubMed
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. - PubMed
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. - PubMed
- Funder JW. Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu Rev Med. 1997;48:231–40. - PubMed
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH085774/MH/NIMH NIH HHS/United States
- R01 MH085774-01A2/MH/NIMH NIH HHS/United States
- R01 MH085774-02/MH/NIMH NIH HHS/United States
- R01 NS061856/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous