Estrogen receptor beta signaling through phosphatase and tensin homolog/phosphoinositide 3-kinase/Akt/glycogen synthase kinase 3 down-regulates blood-brain barrier breast cancer resistance protein - PubMed (original) (raw)
Estrogen receptor beta signaling through phosphatase and tensin homolog/phosphoinositide 3-kinase/Akt/glycogen synthase kinase 3 down-regulates blood-brain barrier breast cancer resistance protein
A M S Hartz et al. J Pharmacol Exp Ther. 2010 Aug.
Abstract
Breast cancer resistance protein (BCRP) is an ATP-driven efflux pump at the blood-brain barrier that limits central nervous system pharmacotherapy. Our previous studies showed rapid loss of BCRP transport activity in rat brain capillaries exposed to low concentrations of 17-beta-estradiol (E2); this occurred without acute change in BCRP protein expression. Here, we describe a pathway through which sustained, extended exposure to E2 signals down-regulation of BCRP at the blood-brain barrier. Six-hour exposure of isolated rat and mouse brain capillaries to E2 reduced BCRP transport activity and BCRP monomer and dimer expression. Experiments with brain capillaries from estrogen receptor (ER)alpha and ERbeta knockout mice and with ER agonists and antagonists showed that E2 signaled through ERbeta to down-regulate BCRP expression. In rat brain capillaries, E2 increased unphosphorylated, active phosphatase and tensin homolog (PTEN); decreased phosphorylated, active Akt; and increased phosphorylated, active glycogen synthase kinase (GSK)3. Consistent with this, inhibition of phosphoinositide 3-kinase (PI3K) or Akt decreased BCRP activity and protein expression, and inhibition of PTEN or GSK3 reversed the E2 effect on BCRP. Lactacystin, a proteasome inhibitor, abolished E2-mediated BCRP down-regulation, suggesting internalization followed by transporter degradation. Dosing mice with E2 reduced BCRP activity in brain capillaries within 1 h; this reduction persisted for 24 h. BCRP protein expression in brain capillaries was unchanged 1 h after E2 dosing but was substantially reduced 6 and 24 h after dosing. Thus, E2 signals through ERbeta, PTEN/PI3K/Akt/GSK3 to stimulate proteasomal degradation of BCRP. These in vitro and in vivo findings imply that E2-mediated down-regulation of blood-brain barrier BCRP has the potential to increase brain uptake of chemotherapeutics that are BCRP substrates.
Figures
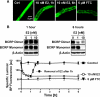
Fig. 1.
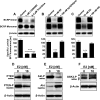
E2-mediated down-regulation of BCRP. A, representative images of isolated rat brain capillaries that were incubated with 2 μM BODIPY FL prazosin for 1 h and then exposed to 10 nM E2 for 1 or 6 h, or to the BCRP inhibitor FTC. Note that E2 decreased luminal BODIPY FL prazosin fluorescence to levels comparable with those observed with BCRP inhibition by FTC. B, Western blots showing BCRP monomer and dimer protein expression in brain capillaries exposed to E2 for 1 and 6 h and time course of BODIPY FL prazosin luminal fluorescence in control-, FTC-, and E2-treated rat brain capillaries. For specific luminal BODIPY FL prazosin (BP) fluorescence, each data point represents the mean ± S.E.M. for 10 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale, 0–255).
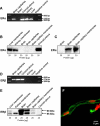
Fig. 2.
Expression of ERα and ERβ in isolated rat brain capillaries. A, RT-PCR for ERα (310-bp amplicon). B, Western blot showing ERα in liver, kidney, and choroid plexus. C, Western blot (longer exposure of blotting membrane) showing ERα in brain capillaries and brain capillary membranes, but not in total brain. D, RT-PCR for ERβ (374-bp amplicon) shows expression in brain capillaries, brain, and choroid plexus. E, ERβ protein expression in kidney, brain, and brain capillaries. Two bands were detected at 55 and 60 kDa (determined by digital molecular weight analysis). F, representative ERβ immunostaining of a brain capillary (green); nuclei were counterstained with propidium iodide (red).
Fig. 3.
E2 signals through ERβ to down-regulate BCRP protein expression and specific transport function. A, ERα activation with 1 nM PPT had no effect on BCRP expression and function within 6 h. B, 100 nM MPP, an ERα antagonist, did not prevent E2-mediated BCRP down-regulation. C, ERβ agonist DPN (10 nM) reduced BCRP expression and transport activity within 6 h. D, blocking ERβ with 1 μM ICI182,780 abolished E2-mediated down-regulation of BCRP. For specific luminal BODIPY FL prazosin (BP) fluorescence, each data point represents the mean ± S.E.M. for 10 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale, 0–255). ∗∗∗, P < 0.001, significantly lower than controls.
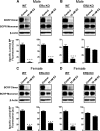
Fig. 4.
E2 down-regulates BCRP in isolated brain capillaries from ERα knockout mice but not ERβ knockout mice. A, E2 decreased BCRP expression and specific transport activity in capillaries isolated from male wild-type and ERα knockout mice. B, E2 had no effect in male ERβ knockout mice. C, in isolated capillaries from female wild-type and ERα knockout mice E2 also down-regulated BCRP. D, as in isolated brain capillaries from male mice, E2 did not down-regulate BCRP protein expression or transport activity in capillaries from female ERβ knockout mice. For specific luminal BODIPY FL prazosin (BP) fluorescence, each data point represents the mean ± S.E.M. for 15 capillaries from a single preparation (pooled tissue from 20 mice per group). Units are arbitrary fluorescence units (scale, 0–255). Statistical comparison: ∗∗∗, P < 0.001, significantly lower than controls.
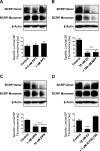
Fig. 5.
E2 signals BCRP down-regulation through PTEN/PI3K/Akt/GSK3. A, PI3K inhibitor LY294002 and the Akt inhibitor triciribine reduced BCRP specific transport activity and BCRP dimer expression in isolated rat brain capillaries. B and C, PTEN inhibitor bpV(HOpic) and the GSK3 inhibitor XIII abolish the E2 effect on BCRP and restore monomer and dimer expression as well as transporter function. D, 6-h exposure of brain capillaries to 10 nM E2 caused a shift from inactive, phosphorylated PTEN to active PTEN. E, E2-mediated activation of PTEN increased the level of inactive Akt and decreased the level of active, phosphorylated Akt. F, E2 also increased the level of active, phosphorylated GSK3-α and GSK3-β. For specific luminal BODIPY FL prazosin (BP) fluorescence, each data point represents the mean ± S.E.M. for 10 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale, 0–255). ∗∗∗, P < 0.001, significantly lower than controls.
Fig. 6.
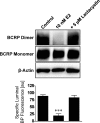
E2-mediated BCRP down-regulation involves proteasomal degradation. The proteasome inhibitor lactacystin abolished E2-mediated down-regulation of BCRP-specific transport activity and dimer expression. For specific luminal BODIPY FL prazosin (BP) fluorescence, each data point represents the mean ± S.E.M. for 10 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale, 0–255). Statistical comparison: ∗∗∗, P < 0.001, significantly lower than controls.
Fig. 7.
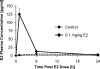
Time course of changes in plasma E2 in mice after a single dose of 0.1 mg/kg E2 given by intraperitoneal injection. Data are given as mean ± S.E.M. (n = 20 mice per group; plasma from each mouse assayed at each time).
Fig. 8.
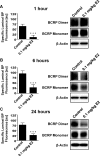
In vivo time course of BCRP protein expression and transport activity in brain capillaries. A to C, dosing mice with 0.1 mg/kg E2 reduced BCRP transport activity in brain capillaries after 1, 6, and 24 h. BCRP protein expression was reduced 6 and 24 h after E2 dosing. For specific luminal BODIPY FL prazosin (BP) fluorescence, each data point represents the mean ± S.E.M. for 10 capillaries from a single preparation (pooled tissue from 10 rats). Units are arbitrary fluorescence units (scale, 0–255). ∗∗∗, P < 0.001, significantly lower than controls.
Fig. 9.
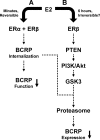
Proposed signaling pathways through which E2 reduces BCRP transport activity and protein expression in brain capillaries. Pathway A was characterized previously (Hartz et al., 2010). Pathway B is described in the present study. We speculate that the two pathways are linked in that transporter internalization must precede proteasomal degradation. It remains to be determined whether PTEN/PI3K/Akt/GSK3 signaling directs internalized BCRP to the proteasome or whether it stimulates ubiquitination and processing of the protein.
Similar articles
- Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression.
Nakanishi T, Ross DD. Nakanishi T, et al. Chin J Cancer. 2012 Feb;31(2):73-99. doi: 10.5732/cjc.011.10320. Epub 2011 Nov 18. Chin J Cancer. 2012. PMID: 22098950 Free PMC article. Review. - BCRP at the blood-brain barrier: genomic regulation by 17β-estradiol.
Mahringer A, Fricker G. Mahringer A, et al. Mol Pharm. 2010 Oct 4;7(5):1835-47. doi: 10.1021/mp1001729. Epub 2010 Sep 9. Mol Pharm. 2010. PMID: 20735085 - 17-β-Estradiol: a powerful modulator of blood-brain barrier BCRP activity.
Hartz AM, Mahringer A, Miller DS, Bauer B. Hartz AM, et al. J Cereb Blood Flow Metab. 2010 Oct;30(10):1742-55. doi: 10.1038/jcbfm.2010.36. Epub 2010 Mar 10. J Cereb Blood Flow Metab. 2010. PMID: 20216549 Free PMC article. - The xenoestrogens ethinylestradiol and bisphenol A regulate BCRP at the blood-brain barrier of rats.
Nickel S, Mahringer A. Nickel S, et al. Xenobiotica. 2014 Nov;44(11):1046-54. doi: 10.3109/00498254.2014.922226. Epub 2014 Jun 19. Xenobiotica. 2014. PMID: 24945792 - The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease.
Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. Oudit GY, et al. J Mol Cell Cardiol. 2004 Aug;37(2):449-71. doi: 10.1016/j.yjmcc.2004.05.015. J Mol Cell Cardiol. 2004. PMID: 15276015 Review.
Cited by
- Regulation of ABC transporters at the blood-brain barrier.
Miller DS. Miller DS. Clin Pharmacol Ther. 2015 Apr;97(4):395-403. doi: 10.1002/cpt.64. Epub 2015 Jan 20. Clin Pharmacol Ther. 2015. PMID: 25670036 Free PMC article. Review. - ABCG2 is associated with HER-2 expression, lymph node metastasis and clinical stage in breast invasive ductal carcinoma.
Xiang L, Su P, Xia S, Liu Z, Wang Y, Gao P, Zhou G. Xiang L, et al. Diagn Pathol. 2011 Sep 27;6:90. doi: 10.1186/1746-1596-6-90. Diagn Pathol. 2011. PMID: 21943250 Free PMC article. - Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair.
Larson TA. Larson TA. Front Endocrinol (Lausanne). 2018 Apr 30;9:205. doi: 10.3389/fendo.2018.00205. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29760681 Free PMC article. Review. - Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression.
Nakanishi T, Ross DD. Nakanishi T, et al. Chin J Cancer. 2012 Feb;31(2):73-99. doi: 10.5732/cjc.011.10320. Epub 2011 Nov 18. Chin J Cancer. 2012. PMID: 22098950 Free PMC article. Review. - Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention.
Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro A, Drobot L, Rakus D, Gizak A, Laidler P, Dulińska-Litewka J, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Montalto G, Cervello M, Fitzgerald TL, Demidenko Z, Martelli AM, Cocco L, Steelman LS, McCubrey JA. Davis NM, et al. Oncotarget. 2014 Jul 15;5(13):4603-50. doi: 10.18632/oncotarget.2209. Oncotarget. 2014. PMID: 25051360 Free PMC article. Review.
References
- Bauer B, Hartz AM, Miller DS. (2007) Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667–675 - PubMed
- Breedveld P, Beijnen JH, Schellens JH. (2006) Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci 27:17–24 - PubMed
- Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. (2009) P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther 330:956–963 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials