Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway - PubMed (original) (raw)
Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway
Puneet Khandelwal et al. EMBO J. 2010.
Abstract
Compensatory endocytosis (CE) ensures recycling of membrane components and maintenance of plasma membrane size; however, the mechanisms, regulation, and physiological functions of clathrin-independent modes of CE are poorly understood. CE was studied in umbrella cells, which undergo regulated exocytosis of subapical discoidal/fusiform vesicles (DFV) during bladder filling, and may then replenish the pool of DFV by internalizing apical membrane during voiding. We found that voiding-stimulated CE, which depended on beta(1) integrin-associated signalling pathways, occurred by a dynamin-, actin-, and RhoA-regulated mechanism and was independent of caveolins, clathrin, and flotillin. Internalized apical membrane and fluid were initially found in ZO-1-positive vesicles, which were distinct from DFV, classical early endosomes, or the Golgi, and subsequently in lysosomes. We conclude that clathrin-independent CE in umbrella cells functions to recover membrane during voiding, is integrin regulated, occurs by a RhoA- and dynamin-dependent pathway, and terminates in degradation and not recapture of membrane in DFV.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
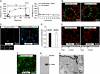
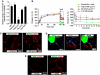
Voiding stimulates CE. (A) Left panel: To simulate bladder filling, uroepithelial tissue was mounted in an Ussing stretch chamber and the mucosal hemichamber was slowly filled until the tissue bowed outwards. At the indicated time, experimental voiding was induced by removing fluid in the mucosal hemichamber until the tissue bowed inwards. The change in _C_T was measured in quiescent tissue (no stretch) or tissue that was bowed outwards and then inwards (control). The boxed region is expanded in the panel on the right. Right panel: Changes in _C_T in response to experimental voiding. The time scale is renumbered so that the initiation of voiding occurs at _t_=0. Values are mean±s.e.m. (_n_⩾7), and those values significantly different than no stretch samples (P<0.05) are indicated with an asterisk. (B) Uptake of FITC-labelled WGA or dextran added to the mucosal hemichamber 10 min before voiding and then processed for immunofluorescence 5 min after experimental voiding. A 3D reconstruction is shown. The junctional complex surrounding individual umbrella cells is indicated by arrows. F-actin associated with the apical plasma membrane is difficult to discern in these images, but is apparent along the lateral surfaces of the umbrella cells. The scattered patches of actin observed at the base of the cells are the cortical actin associated with the plasma membrane of the underlying intermediate cell layer. The distribution of markers near the junctions is more apparent in Supplementary movies S1 and S2. (C) Distribution of FITC-dextran and Alexa647-WGA co-internalized for 10 or 90 min after experimental voiding. The boxed region in the left-hand panel is magnified in images below. (D) Co-localization coefficient for the fraction of FITC-dextran that co-localized with Alexa647-WGA. Values are mean±s.e.m. (_n_⩾7). (E) Uptake of FITC-WGA added 5 min after voiding and then incubated for 15 or 45 min. (F) Distribution of FITC-WGA added to the apical surface of the umbrella cells and then fixed and visualized. (G) Membrane fractions of uroepithelium were incubated with agarose-WGA in the presence (+) or absence (−) of _N_-acetyl glucosamine (GlcNAc). The bound proteins were resolved by SDS–PAGE and UP3a detected by western blot analysis. (H) TEM of rat bladder filled with cationized ferritin and induced to void. Left panel: DFV are indicated with arrows and a cationized ferritin-positive PJAE is indicated with an asterisk. The boxed PJAE is magnified in the inset. Right panels: examples of cationized ferritin-positive PJAEs.
Figure 2
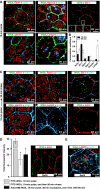
Delivery of endocytosed membrane to late endosomes/lysosomes. (A) Co-localization of EEA1, Rab11a, ZO-1, giantin, or LAMP2 with FITC-WGA 10 min after experimental voiding. (B) Co-localization coefficients for FITC-WGA and the indicated marker 10 or 90 min after experimental voiding. Values are mean±s.e.m. (_n_⩾7). Those values significantly different from the 10-min time point (P<0.05) are indicated with an asterisk. (C) Distribution of FITC-WGA and indicated markers 90 min after voiding. (D) Left panel: Intensity of labelled WGA pulsed for 10 min after experimental voiding ± an 80-min chase in the absence of additional marker. Alternatively, WGA was pulsed for 10 min after voiding, chased for 30 min in the absence of filling, and then chased for an additional 60 min during which time the chamber was re-filled. Values are mean±s.e.m. (_n_⩾7) and those values significantly different than samples fixed after the pulse (P<0.05) are indicated with an asterisk. Right panel: Distribution of Alexa488-WGA that was internalized for 10 min after voiding, chased for 30 min, and then chased for 60 min during which time the mucosal hemichamber was re-filled. (E) Co-localization of Alexa488-WGA and LAMP2 in tissue that was refilled.
Figure 3
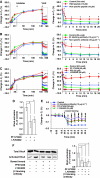
CE is through a caveolin-, clathrin-, and flotillin-independent mechanism. (A) Distribution of the α adaptin of AP-2, clathrin heavy chain (CHC), caveolin-1, -2, or flotillin-1 in cryosections of uroepithelial tissue. The location of an umbrella cell apical membrane is indicated by arrows. (B) Analysis of the number of clathrin-coated pits (CCP) and flask-shaped invaginations (FSI) per micron of the indicated membrane in samples fixed before voiding or fixed immediately after voiding. Values are mean±s.e.m. (_n_=25). Panels to the right are electron micrographs of the sampled membranes. CCPs or FSIs are indicated with arrows. (C) Distribution of the α adaptin of AP-2 and caveolin-1 in tissue that was experimentally filled and then fixed 5-min after voiding. (D) Effect of chlorpromazine (10 μg/ml) or K+-free buffer on _C_T responses during experimental filling and voiding. The boxed region in the upper panel is expanded in the lower panel. Induction of voiding is indicated by the arrow. Control data are reproduced from Figure 1A. Values are mean±s.e.m. (_n_⩾7), and those values significantly different than control samples (P<0.05) are indicated with an asterisk.
Figure 4
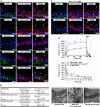
Dependence of CE on dynamin. (A) Localization of dynamin-2 in uroepithelial tissue. The apical surface of an umbrella cell is indicated by arrows. (B) Effect of dynasore on _C_T during filling and voiding. Voiding was initiated at the arrow. The boxed region in the left panel is expanded in the right-hand panel. Control data are reproduced from Figure 1A. Values are mean±s.e.m. (_n_⩾5), and those values significantly different than control samples (P<0.5) are indicated with an asterisk. (C) Uptake of FITC-WGA and FITC-dextran in tissue treated with dynasore (250 μM). (D) Quantitation of FITC-WGA and FITC-dextran uptake in tissue treated with MβCD (10 mM), cyto-D (25 μg/ml), C3 toxin (1 μg/ml), dynasore (250 μM), or Y-27632 (25 μM). Values are mean±s.e.m. (_n_⩾10), and those values significantly different than control samples (P<0.05) are indicated with an asterisk. (E, F) Rat bladders were transduced in situ with adenovirus expressing GFP alone or GFP-labelled DN-dynaminK44A (DN-dynamin). The bladder was filled with Alexa647-WGA, stimulated to undergo voiding, excised, and then fixed and processed for immunofluorescence. (E) A transduced cell expressing DN-dynaminK44A (K44A) is indicated with an asterisk. (F) The uptake of Alex647-WGA was quantified in cells expressing GFP or DN-dynaminK44A (K44A) and normalized to uptake in adjacent cells that did not express exogenous protein. Data are mean±s.e.m. (_n_⩾70).
Figure 5
CE is actin and cholesterol dependent. (A) Changes in _C_T in control tissue or that treated with cytoD (25 μg/ml). Voiding was induced at the arrow. The boxed region is expanded in the right-hand panel. Control data are reproduced from Figure 1A. Values are mean±s.e.m. (_n_⩾5), and those values significantly different than control samples (P<0.05) are indicated with an asterisk. (B) Uptake of FITC-WGA or FITC-dextran in tissue treated with cytoD. (C) Effect of MβCD treatment on umbrella cell detergent-resistant membranes. The apical surface of umbrella cells was incubated in the presence or absence of MβCD, and Triton X-100-resistant (floating membrane) and -soluble membranes resolved by sucrose flotation gradients. The localization of UP3a or flotillin in the gradients was determined by western blotting. (D) Association of HRP-WGA with floating and soluble membrane fractions (±MβCD treatment). Data are mean±s.e.m. (_n_=3). (E) Uptake of FITC-WGA or FITC-dextran in tissue treated with MβCD.
Figure 6
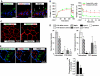
RhoA dependence of CE in umbrella cells. (A) Uptake of Alexa647-WGA was quantified in cells expressing GFP or the indicated ras family GTPase and normalized to uptake in adjacent cells that did not express exogenous protein. GFP data is reproduced from Figure 4F. Data are mean±s.e.m. (_n_⩾70). (B) Effect of C3 toxin (1 μg/ml), toxin B (40 ng/ml), and Y-27632 (25 μM) on changes in _C_T. Voiding was induced at the arrow. The boxed region in the left-hand panel is expanded in the right-hand panel. Control data are reproduced from Figure 1A. Values are mean±s.e.m. (_n_⩾5), and those values significantly different than controls (P<0.05) are indicated with an asterisk. (C) Internalization of FITC-WGA or FITC-dextran in C3 toxin-treated cells. (D) Uptake of Alexa647-WGA in rat umbrella cells transduced in situ with GFP and dominant-negative RhoA (transduced cell is marked with an asterisk). (E) Effect of Y-27632 on uptake of FITC-WGA or FITC-dextran after experimental voiding.
Figure 7
Function of β1 integrin and associated signalling pathways in voiding-induced CE. (A) Changes in _C_T for tissue treated with RGD peptide (10 μM) or non-specific peptide (10 μM). (B) Effect of β1-blocking antibody (10 μg/ml) or non-specific antibody (10 μg/ml) on changes in _C_T. (C) Changes in _C_T for tissue treated with PF573228 (20 μM), PP2 (25 μM), or LY294002 (50 μM). (A–C) Inhibitor was added at the indicated time. Voiding was induced at the arrow and the boxed region in the left-hand panel is expanded in the right-hand panel. Control data are reproduced from Figure 1A. Values are mean±s.e.m. (_n_⩾5), and those values significantly different than controls (P<0.05) are indicated with an asterisk. (D) Effect of PF573228 or LY294002 on RhoA activation in intact bladders. Values are mean±s.e.m. (_n_=3) and those values significantly different than control samples (P<0.05) are indicated with an asterisk. (E) Changes in _C_T in response to inwards bowing. Tissue was left untreated (control) or treated with blocking antibody, PF573228, or LY294002. Values are mean±s.e.m. (_n_⩾5), and those values significantly different than control samples (P<0.05) are indicated with an asterisk (PF573228) or a closed circle (LY294002). (F) Left panel: Total and activated RhoA in control tissue or that treated with blocking antibody, PF573228, or LY294002. Right panel: RhoA activation expressed as a per cent of control. Values are mean±s.e.m. (_n_=6), and those values significantly different than control samples (P<0.05) are indicated with an asterisk.
Similar articles
- ARF1 is directly involved in dynamin-independent endocytosis.
Kumari S, Mayor S. Kumari S, et al. Nat Cell Biol. 2008 Jan;10(1):30-41. doi: 10.1038/ncb1666. Epub 2007 Dec 16. Nat Cell Biol. 2008. PMID: 18084285 Free PMC article. - Expansion and contraction of the umbrella cell apical junctional ring in response to bladder filling and voiding.
Eaton AF, Clayton DR, Ruiz WG, Griffiths SE, Rubio ME, Apodaca G. Eaton AF, et al. Mol Biol Cell. 2019 Jul 22;30(16):2037-2052. doi: 10.1091/mbc.E19-02-0115. Epub 2019 Jun 5. Mol Biol Cell. 2019. PMID: 31166831 Free PMC article. - Cargo-specific recruitment in clathrin- and dynamin-independent endocytosis.
Moreno-Layseca P, Jäntti NZ, Godbole R, Sommer C, Jacquemet G, Al-Akhrass H, Conway JRW, Kronqvist P, Kallionpää RE, Oliveira-Ferrer L, Cervero P, Linder S, Aepfelbacher M, Zauber H, Rae J, Parton RG, Disanza A, Scita G, Mayor S, Selbach M, Veltel S, Ivaska J. Moreno-Layseca P, et al. Nat Cell Biol. 2021 Oct;23(10):1073-1084. doi: 10.1038/s41556-021-00767-x. Epub 2021 Oct 6. Nat Cell Biol. 2021. PMID: 34616024 Free PMC article. - Membrane lipids and proteins as modulators of urothelial endocytic vesicles pathways.
Grasso EJ, Calderón RO. Grasso EJ, et al. Histochem Cell Biol. 2013 Nov;140(5):507-20. doi: 10.1007/s00418-013-1095-8. Epub 2013 Apr 27. Histochem Cell Biol. 2013. PMID: 23624723 Review. - Lipid rafts, caveolae, and their endocytosis.
Lajoie P, Nabi IR. Lajoie P, et al. Int Rev Cell Mol Biol. 2010;282:135-63. doi: 10.1016/S1937-6448(10)82003-9. Epub 2010 Jun 18. Int Rev Cell Mol Biol. 2010. PMID: 20630468 Review.
Cited by
- Roles of rho GTPases in intracellular transport and cellular transformation.
Chi X, Wang S, Huang Y, Stamnes M, Chen JL. Chi X, et al. Int J Mol Sci. 2013 Mar 28;14(4):7089-108. doi: 10.3390/ijms14047089. Int J Mol Sci. 2013. PMID: 23538840 Free PMC article. Review. - MAL facilitates the incorporation of exocytic uroplakin-delivering vesicles into the apical membrane of urothelial umbrella cells.
Zhou G, Liang FX, Romih R, Wang Z, Liao Y, Ghiso J, Luque-Garcia JL, Neubert TA, Kreibich G, Alonso MA, Schaeren-Wiemers N, Sun TT. Zhou G, et al. Mol Biol Cell. 2012 Apr;23(7):1354-66. doi: 10.1091/mbc.E11-09-0823. Epub 2012 Feb 9. Mol Biol Cell. 2012. PMID: 22323295 Free PMC article. - CD103 (αE Integrin) Undergoes Endosomal Trafficking in Human Dendritic Cells, but Does Not Mediate Epithelial Adhesion.
Swain S, Roe MM, Sebrell TA, Sidar B, Dankoff J, VanAusdol R, Smythies LE, Smith PD, Bimczok D. Swain S, et al. Front Immunol. 2018 Dec 21;9:2989. doi: 10.3389/fimmu.2018.02989. eCollection 2018. Front Immunol. 2018. PMID: 30622531 Free PMC article. - Invasion of Host Cells and Tissues by Uropathogenic Bacteria.
Lewis AJ, Richards AC, Mulvey MA. Lewis AJ, et al. Microbiol Spectr. 2016 Dec;4(6):10.1128/microbiolspec.UTI-0026-2016. doi: 10.1128/microbiolspec.UTI-0026-2016. Microbiol Spectr. 2016. PMID: 28087946 Free PMC article. Review. - The umbrella cell keratin network: organization as a tile-like mesh, formation of a girded layer in response to bladder filling, and dependence on the plectin cytolinker.
Ruiz WG, Clayton DR, Parakala-Jain T, Dalghi MG, Franks J, Apodaca G. Ruiz WG, et al. bioRxiv [Preprint]. 2024 Jun 13:2024.06.11.598498. doi: 10.1101/2024.06.11.598498. bioRxiv. 2024. PMID: 38915686 Free PMC article. Updated. Preprint.
References
- Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE (2004) Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res 301: 23–30 - PubMed
- Amano O, Kataoka S, Yamamoto T (1991) Turnover of asymmetric unit membranes in the transitional epithelial superficial cells of the rat urinary bladder. Anatom Record 229: 9–15 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK077777/DK/NIDDK NIH HHS/United States
- P30DK079307/DK/NIDDK NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- R37-DK54425/DK/NIDDK NIH HHS/United States
- R37 DK054425/DK/NIDDK NIH HHS/United States
- R01 DK077777/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases