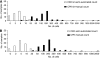Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer - PubMed (original) (raw)
Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer
L M Flores et al. Br J Cancer. 2010.
Abstract
Background: Circulating tumour cells (CTCs) offer a non-invasive approach to obtain and characterise metastatic tumour cells, but their usefulness has been limited by low CTC yields from conventional isolation methods.
Methods: To improve CTC yields and facilitate their molecular characterisation we compared the Food and Drug Administration-approved CellSearch Epithelial Kit (CEK) to a simplified CTC capture method, CellSearch Profile Kit (CPK), on paired blood samples from patients with metastatic breast (n=75) and lung (n=71) cancer. Molecular markers including Human Epidermal growth factor Receptor 2 (HER2) were evaluated on CTCs by fluorescence in situ hybridisation (FISH) and compared to patients' primary and metastatic cancer.
Results: The median cell count from patients with breast cancer using the CPK was 117 vs 4 for CEK (P<0.0001). Lung cancer samples were similar; CPK: 145 cells vs CEK:4 cells (P<0.0001). Recovered CTCs were relatively pure (60-70%) and were evaluable by FISH and immunofluorescence. A total of 10 of 30 (33%) breast cancer patients with HER2-negative primary and metastatic tissue had HER2-amplified CTCs.
Conclusion: The CPK method provides a high yield of relatively pure CTCs, facilitating their molecular characterisation. Circulating tumour cells obtained using CPK technology demonstrate that significant discordance exists between HER2 amplification of a patient's CTCs and that of the primary and metastatic tumour.
Figures
Figure 1
CPK method improves cell yields over CEK method. The blood samples from patients with (A) breast cancer (_n_=75) or (B) NSCLC (_n_=71) processed in parallel by the CEK method with semi-automated quantification (open columns) or the CPK method with manual quantification (closed columns).
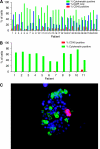
Figure 2
The CPK method isolates a highly enriched population of CTCs. Percentage of total cells captured by the CPK method from patients with NSCLC, staining for cytokeratin (AE1/AE3), CD45, or DAPI nuclear stain alone by (A) immunofluorescence or (B) FACS. Similar results were seen with samples from patients with breast cancer. (C) Representative immunofluorescence image of CPK-captured cells from patient with NSCLC, labelled with cytokeratin (green), DAPI (blue), and CD45 (red).
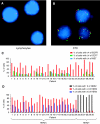
Figure 3
FISH analysis confirms that samples processed by the CPK method have a low percentage of contaminating normal cells. Representative FISH images of cells processed by CPK method, (A) lymphocyte with two copies of CEP7 (green), EGFR (red), and MET (blue), (B) CTC with amplified EGFR and MET. (C) Percentage of total cells captured by the CPK method from patients with NSCLC with ⩾4 copies of EGFR, CEP7, and MET per nucleus. (D) Percentage of total cells captured by the CPK method from patients with clinically defined HER2-positive (patient number 1–24) or HER2-negative (patient number 25–30) breast cancer, with the indicated copies of HER2 per nucleus.
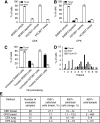
Figure 4
Apoptotic CTCs are less effectively captured by CEK or CPK methods. SKBR3 (HER2+ breast cancer) or HCC827 (EGFR mutant NSCLC) cells treated with vehicle or tyrosine kinase inhibitor (SKBR3: 1 _μ_M lapatinib and HCC827; 1 _μ_M gefitinib) for 24 h and processed with (A) CEK method. (B) CPK method or (C) smeared directly on slide without processing. Plots depict percentage of cells (±1 s.d.) staining for Ki67 (open bars, proliferation marker) or TUNEL (closed bars, apoptosis marker) by immunohistochemistry. (D) Percentage of CPK-processed CTCs from patients with NSCLC staining positive for KI67 and TUNEL. (E) Comparison of KI67 expression in CTCs recovered by CEK or CPK methods.
Similar articles
- Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients.
Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, Cappadona S, Biganzoli L, Giannini A, Di Leo A. Pestrin M, et al. Breast Cancer Res Treat. 2009 Dec;118(3):523-30. doi: 10.1007/s10549-009-0461-7. Epub 2009 Jul 12. Breast Cancer Res Treat. 2009. PMID: 19597704 - A novel method for downstream characterization of breast cancer circulating tumor cells following CellSearch isolation.
Frithiof H, Welinder C, Larsson AM, Rydén L, Aaltonen K. Frithiof H, et al. J Transl Med. 2015 Apr 21;13:126. doi: 10.1186/s12967-015-0493-1. J Transl Med. 2015. PMID: 25896421 Free PMC article. - Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer.
Krishnamurthy S, Bischoff F, Ann Mayer J, Wong K, Pham T, Kuerer H, Lodhi A, Bhattacharyya A, Hall C, Lucci A. Krishnamurthy S, et al. Cancer Med. 2013 Apr;2(2):226-33. doi: 10.1002/cam4.70. Epub 2013 Mar 6. Cancer Med. 2013. PMID: 23634290 Free PMC article. - The significance of circulating tumour cells in breast cancer: a review.
Castle J, Shaker H, Morris K, Tugwood JD, Kirwan CC. Castle J, et al. Breast. 2014 Oct;23(5):552-60. doi: 10.1016/j.breast.2014.07.002. Epub 2014 Aug 11. Breast. 2014. PMID: 25124235 Review. - Emerging technologies for CTC detection based on depletion of normal cells.
Lustberg M, Jatana KR, Zborowski M, Chalmers JJ. Lustberg M, et al. Recent Results Cancer Res. 2012;195:97-110. doi: 10.1007/978-3-642-28160-0_9. Recent Results Cancer Res. 2012. PMID: 22527498 Free PMC article. Review.
Cited by
- Does tumour dormancy offer a therapeutic target?
Goss PE, Chambers AF. Goss PE, et al. Nat Rev Cancer. 2010 Dec;10(12):871-7. doi: 10.1038/nrc2933. Epub 2010 Nov 4. Nat Rev Cancer. 2010. PMID: 21048784 Review. - Clinical relevance and biology of circulating tumor cells.
Bednarz-Knoll N, Alix-Panabières C, Pantel K. Bednarz-Knoll N, et al. Breast Cancer Res. 2011;13(6):228. doi: 10.1186/bcr2940. Epub 2011 Nov 1. Breast Cancer Res. 2011. PMID: 22114869 Free PMC article. Review. - Circulating tumors cells as biomarkers: progress toward biomarker qualification.
Danila DC, Pantel K, Fleisher M, Scher HI. Danila DC, et al. Cancer J. 2011 Nov-Dec;17(6):438-50. doi: 10.1097/PPO.0b013e31823e69ac. Cancer J. 2011. PMID: 22157288 Free PMC article. Review. - Analytical validation of the CellMax platform for early detection of cancer by enumeration of rare circulating tumor cells.
Gupta P, Gulzar Z, Hsieh B, Lim A, Watson D, Mei R. Gupta P, et al. J Circ Biomark. 2019 Dec 31;8:1849454419899214. doi: 10.1177/1849454419899214. eCollection 2019 Jan-Dec. J Circ Biomark. 2019. PMID: 31921364 Free PMC article. - Mesenchymal Characteristics and Predictive Biomarkers on Circulating Tumor Cells for Therapeutic Strategy.
Okabe T, Togo S, Fujimoto Y, Watanabe J, Sumiyoshi I, Orimo A, Takahashi K. Okabe T, et al. Cancers (Basel). 2020 Nov 30;12(12):3588. doi: 10.3390/cancers12123588. Cancers (Basel). 2020. PMID: 33266262 Free PMC article. Review.
References
- Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Gottert J, McCarley RL, Nikitopoulos D, Murphy MC, Soper SA (2008) Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J Am Chem Soc 130: 8633–8641 - PMC - PubMed
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10: 6897–6904 - PubMed
- Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, Hayes DF (2006) Circulating tumor cells versus imaging – predicting overall survival in metastatic breast cancer. Clin Cancer Res 12: 6403–6409 - PubMed
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781–791 - PubMed
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12: 4218–4224 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous