A comparison of bacterial composition in diabetic ulcers and contralateral intact skin - PubMed (original) (raw)
A comparison of bacterial composition in diabetic ulcers and contralateral intact skin
Viktoria Gontcharova et al. Open Microbiol J. 2010.
Abstract
An extensive portion of the healthcare budget is allocated to chronic human infection. Chronic wounds in particular are a major contributor to this financial burden. Little is known about the types of bacteria which may contribute to the chronicity, biofilm and overall bioburden of the wound itself. In this study we compare the bacteriology of wounds and associated intact skin. Wound and paired intact skin swabs (from a contralateral location) were collected. The bacterial diversity was determined using bacterial Tag-encoded FLX amplicon pyrosequencing (bTEFAP). Diversity analysis showed intact skin to be significantly more diverse than wounds on both the species and genus levels (3% and 5% divergence). Furthermore, wounds show heightened levels of anaerobic bacteria, like Peptoniphilus, Finegoldia, and Anaerococcus, and other detrimental genera such as Corynebacterium and Staphylococcus. Although some of these and other bacterial genera were found to be common between intact skin and wounds, notable opportunistic wound pathogens were found at lower levels in intact skin. Principal Component Analysis demonstrated a clear separability of the two groups. The findings of the study not only greatly support the hypothesis of differing bacterial composition of intact skin and wounds, but also contribute additional insight into the ecology of skin and wound microflora. The increased diversity and lowered levels of opportunistic pathogens found in skin make the system highly distinguishable from wounds.
Keywords: Biofilm; bTEFAP; bacteria; chronic wounds; diversity; microbiome.; pyrosequencing; skin.
Figures
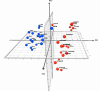
Fig. (1)
PCA for intact skin and wound data. The figure display the three main principal components to which the data was reduced to. The axes represent the values for principal components 1, 2 and 3. Points lying in the negative portion of an axis indicate a negative correlation between the principal component and the sample. The two groups are denoted by different colors to demonstrate the separation between classes (intact skin vs. wounds). The ability to linearly separate the classes within the PCA figures indicates intact skin samples are different from wound samples.
Fig. (2)
Hierarchical clustering of healthy skin and wound data. This figure provides further support for the separability between the two classes of samples, healthy skin and wounds. The figure also indicates low correlation between healthy skin and wound samples for the same individual, indicating lack of contamination or other factors possibly affecting bacterial similarity.
Fig. (3)
Correlation Distances for paired intact skin against wound samples. Pearson Correlation distances, ranging from 0 to 2 were normalized to a scale of 0 to 1 where 1 represents the furthest distance, or the least similar samples.
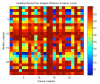
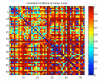
Fig. (4)
Correlation Distances for all against all samples. Pearson Correlation distances, ranging from 0 to 2 were normalized to a scale of 0 to 1 where 1 represents the furthest distance, or the least similar samples.
Similar articles
- Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP).
Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Dowd SE, et al. PLoS One. 2008 Oct 3;3(10):e3326. doi: 10.1371/journal.pone.0003326. PLoS One. 2008. PMID: 18833331 Free PMC article. - High Levels of Oxidative Stress Create a Microenvironment That Significantly Decreases the Diversity of the Microbiota in Diabetic Chronic Wounds and Promotes Biofilm Formation.
Kim JH, Ruegger PR, Lebig EG, VanSchalkwyk S, Jeske DR, Hsiao A, Borneman J, Martins-Green M. Kim JH, et al. Front Cell Infect Microbiol. 2020 Jun 3;10:259. doi: 10.3389/fcimb.2020.00259. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32582564 Free PMC article. - Evaluation of the bacterial diversity of pressure ulcers using bTEFAP pyrosequencing.
Smith DM, Snow DE, Rees E, Zischkau AM, Hanson JD, Wolcott RD, Sun Y, White J, Kumar S, Dowd SE. Smith DM, et al. BMC Med Genomics. 2010 Sep 21;3:41. doi: 10.1186/1755-8794-3-41. BMC Med Genomics. 2010. PMID: 20854691 Free PMC article. - Bacterial Contribution in Chronicity of Wounds.
Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Rahim K, et al. Microb Ecol. 2017 Apr;73(3):710-721. doi: 10.1007/s00248-016-0867-9. Epub 2016 Oct 14. Microb Ecol. 2017. PMID: 27742997 Review. - The Impact of Microbial Communities on Wound Healing: A Review.
Xu Z, Hsia HC. Xu Z, et al. Ann Plast Surg. 2018 Jul;81(1):113-123. doi: 10.1097/SAP.0000000000001450. Ann Plast Surg. 2018. PMID: 29746280 Review.
Cited by
- Next Steps: Studying Diabetic Foot Infections with Next-Generation Molecular Assays.
Sande C, Boston ZJ, Kalan LR, Brennan MB. Sande C, et al. Curr Infect Dis Rep. 2023 Dec;25(12):323-330. doi: 10.1007/s11908-023-00822-8. Epub 2023 Oct 27. Curr Infect Dis Rep. 2023. PMID: 39055239 Free PMC article. - P. aeruginosa interactions with other microbes in biofilms during co-infection.
Oliveira M, Cunha E, Tavares L, Serrano I. Oliveira M, et al. AIMS Microbiol. 2023 Aug 10;9(4):612-646. doi: 10.3934/microbiol.2023032. eCollection 2023. AIMS Microbiol. 2023. PMID: 38173971 Free PMC article. Review. - Effects of Factors Influencing Scar Formation on the Scar Microbiome in Patients with Burns.
Jung Y, Cui HS, Lee EK, Joo SY, Seo CH, Cho YS. Jung Y, et al. Int J Mol Sci. 2023 Nov 6;24(21):15991. doi: 10.3390/ijms242115991. Int J Mol Sci. 2023. PMID: 37958976 Free PMC article. - Therapeutic role of growth factors in treating diabetic wound.
Zheng SY, Wan XX, Kambey PA, Luo Y, Hu XM, Liu YF, Shan JQ, Chen YW, Xiong K. Zheng SY, et al. World J Diabetes. 2023 Apr 15;14(4):364-395. doi: 10.4239/wjd.v14.i4.364. World J Diabetes. 2023. PMID: 37122434 Free PMC article. Review. - Colonizing microbiota is associated with clinical outcomes in diabetic wound healing.
Wang G, Lin Z, Li Y, Chen L, Reddy SK, Hu Z, Garza LA. Wang G, et al. Adv Drug Deliv Rev. 2023 Mar;194:114727. doi: 10.1016/j.addr.2023.114727. Epub 2023 Feb 8. Adv Drug Deliv Rev. 2023. PMID: 36758858 Free PMC article. Review.
References
- Bjarnsholt T, Kirketerp-Moller K, Jensen PO, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10. - PubMed
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45. - PubMed
- Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care. 2008;17:145-2–154. - PubMed
- James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. - PubMed
- Percival SL, Bowler P, Woods EJ. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen. 2008;16:52–7. - PubMed
LinkOut - more resources
Full Text Sources