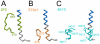Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions - PubMed (original) (raw)
Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions
Victor Buzon et al. PLoS Pathog. 2010.
Abstract
The HIV-1 envelope glycoprotein (Env) composed of the receptor binding domain gp120 and the fusion protein subunit gp41 catalyzes virus entry and is a major target for therapeutic intervention and for neutralizing antibodies. Env interactions with cellular receptors trigger refolding of gp41, which induces close apposition of viral and cellular membranes leading to membrane fusion. The energy released during refolding is used to overcome the kinetic barrier and drives the fusion reaction. Here, we report the crystal structure at 2 A resolution of the complete extracellular domain of gp41 lacking the fusion peptide and the cystein-linked loop. Both the fusion peptide proximal region (FPPR) and the membrane proximal external region (MPER) form helical extensions from the gp41 six-helical bundle core structure. The lack of regular coiled-coil interactions within FPPR and MPER splay this end of the structure apart while positioning the fusion peptide towards the outside of the six-helical bundle and exposing conserved hydrophobic MPER residues. Unexpectedly, the section of the MPER, which is juxtaposed to the transmembrane region (TMR), bends in a 90 degrees-angle sideward positioning three aromatic side chains per monomer for membrane insertion. We calculate that this structural motif might facilitate the generation of membrane curvature on the viral membrane. The presence of FPPR and MPER increases the melting temperature of gp41 significantly in comparison to the core structure of gp41. Thus, our data indicate that the ordered assembly of FPPR and MPER beyond the core contributes energy to the membrane fusion reaction. Furthermore, we provide the first structural evidence that part of MPER will be membrane inserted within trimeric gp41. We propose that this framework has important implications for membrane bending on the viral membrane, which is required for fusion and could provide a platform for epitope and lipid bilayer recognition for broadly neutralizing gp41 antibodies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
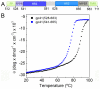
Figure 1. FPPR and MPER increase the melting temperature of gp41.
A) Schematic overview of gp41; FP, fusion peptide; FPPR, fusion peptide proximal region; HR1, heptad repeat 1; HR2, heptad repeat 2; MPER, membrane proximal external region; TMR, transmembrane region. B) Unfolding of gp41528–683 and gp41541–665 monitored by circular dichroism spectroscopy at 222 nm.
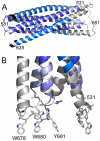
Figure 2. Crystal structure of gp41528–683 reveals a 90 Å long rod-like structure.
A) Ribbon representation of gp41. The previously determined core is colored dark blue (HR1) and marine blue (HR2). The flag sequence present at the N-terminus of HR2 is shown in black. FPPR is colored in light blue and MPER in grey. Note that the N-terminus of FPPR (residue 531) points towards the outside of the rod. B) Close up of the MPER and FPPR region shows the exposure of aromatic side chains Trp 678, Trp 680 and Tyr 681 towards the membrane.
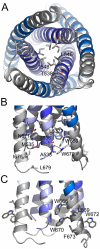
Figure 3. The FPPR-MPER regions are splayed apart.
A) Close-up view from the bottom showing residue Leu 545 as the last coiled coil interacting residue of the HR1 core of gp41. The preceding potential heptad positions are Ala 541 and Thr 538. B) Close up view revealing mostly hydrophobic interactions between FPPR and MPER and only one hydrogen bond between the carbonyl of Ala and NE1 of Trp 670. C) Close-up of solvent exposed hydrophobic MPER residues.
Figure 4. Comparison of MPER conformations.
MPER conformations as determined in complex with broadly neutralizing antibodies (A) 2F5 , (B) Z13e1 and (C) 4E10 are shown in comparison to MPER within trimeric gp41. The corresponding MPER segments are colored equally and residues contacting the 4E10 Fab are shown as sticks. (blue, HR2).
Similar articles
- Immunogens Modeling a Fusion-Intermediate Conformation of gp41 Elicit Antibodies to the Membrane Proximal External Region of the HIV Envelope Glycoprotein.
Vassell R, He Y, Vennakalanti P, Dey AK, Zhuang M, Wang W, Sun Y, Biron-Sorek Z, Srivastava IK, LaBranche CC, Montefiori DC, Barnett SW, Weiss CD. Vassell R, et al. PLoS One. 2015 Jun 18;10(6):e0128562. doi: 10.1371/journal.pone.0128562. eCollection 2015. PLoS One. 2015. PMID: 26087072 Free PMC article. - Fully hydrophobic HIV gp41 adopts a hemifusion-like conformation in phospholipid bilayers.
Lee M, Morgan CA, Hong M. Lee M, et al. J Biol Chem. 2019 Oct 4;294(40):14732-14744. doi: 10.1074/jbc.RA119.009542. Epub 2019 Aug 13. J Biol Chem. 2019. PMID: 31409642 Free PMC article. - Cholesterol-dependent membrane fusion induced by the gp41 membrane-proximal external region-transmembrane domain connection suggests a mechanism for broad HIV-1 neutralization.
Apellániz B, Rujas E, Carravilla P, Requejo-Isidro J, Huarte N, Domene C, Nieva JL. Apellániz B, et al. J Virol. 2014 Nov;88(22):13367-77. doi: 10.1128/JVI.02151-14. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210180 Free PMC article. - Neutralizing Antibodies Targeting HIV-1 gp41.
Caillat C, Guilligay D, Sulbaran G, Weissenhorn W. Caillat C, et al. Viruses. 2020 Oct 23;12(11):1210. doi: 10.3390/v12111210. Viruses. 2020. PMID: 33114242 Free PMC article. Review. - Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.
Cerutti N, Loredo-Varela JL, Caillat C, Weissenhorn W. Cerutti N, et al. Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
Cited by
- Kinetically coupled folding of a single HIV-1 glycoprotein 41 complex in viral membrane fusion and inhibition.
Jiao J, Rebane AA, Ma L, Gao Y, Zhang Y. Jiao J, et al. Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):E2855-64. doi: 10.1073/pnas.1424995112. Epub 2015 May 18. Proc Natl Acad Sci U S A. 2015. PMID: 26038562 Free PMC article. - Mechanistic insights of host cell fusion of SARS-CoV-1 and SARS-CoV-2 from atomic resolution structure and membrane dynamics.
Chakraborty H, Bhattacharjya S. Chakraborty H, et al. Biophys Chem. 2020 Oct;265:106438. doi: 10.1016/j.bpc.2020.106438. Epub 2020 Jul 22. Biophys Chem. 2020. PMID: 32721790 Free PMC article. Review. - Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies.
Kwong PD, Mascola JR. Kwong PD, et al. Immunity. 2012 Sep 21;37(3):412-25. doi: 10.1016/j.immuni.2012.08.012. Immunity. 2012. PMID: 22999947 Free PMC article. Review. - Regulation of epitope exposure in the gp41 membrane-proximal external region through interactions at the apex of HIV-1 Env.
Schapiro HM, Khasnis MD, Ahn K, Karagiaridi A, Hayden S, Cilento ME, Root MJ. Schapiro HM, et al. PLoS Pathog. 2022 May 18;18(5):e1010531. doi: 10.1371/journal.ppat.1010531. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35584191 Free PMC article. - Roles of conserved tryptophans in trimerization of HIV-1 membrane-proximal external regions: Implications for virucidal design via alchemical free-energy molecular simulations.
Gossert ST, Parajuli B, Chaiken I, Abrams CF. Gossert ST, et al. Proteins. 2018 Jul;86(7):707-711. doi: 10.1002/prot.25504. Epub 2018 Apr 19. Proteins. 2018. PMID: 29633345 Free PMC article.
References
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. - PubMed
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, B R. The HIV Env-mediated fusion reaction. Biochimica Biophysica Acta. 2003;1614:36–50. - PubMed
- Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. - PubMed
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources