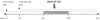Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats - PubMed (original) (raw)
. 2010 May 6;5(5):e10507.
doi: 10.1371/journal.pone.0010507.
Dhyana Sankar, Nan Li, Emily Williams, Kin-Kwan Lai, Asmaa Sayed Abdelgeliel, Claudio F Gonzalez, Clive H Wasserfall, Joseph Larkin, Desmond Schatz, Mark A Atkinson, Eric W Triplett, Josef Neu, Graciela L Lorca
Affiliations
- PMID: 20463897
- PMCID: PMC2865539
- DOI: 10.1371/journal.pone.0010507
Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats
Ricardo Valladares et al. PLoS One. 2010.
Abstract
Background: The intestinal epithelium is a barrier that composes one of the most immunologically active surfaces of the body due to constant exposure to microorganisms as well as an infinite diversity of food antigens. Disruption of intestinal barrier function and aberrant mucosal immune activation have been implicated in a variety of diseases within and outside of the gastrointestinal tract. With this model in mind, recent studies have shown a link between diet, composition of intestinal microbiota, and type 1 diabetes pathogenesis. In the BioBreeding rat model of type 1 diabetes, comparison of the intestinal microbial composition of diabetes prone and diabetes resistant animals found Lactobacillus species were negatively correlated with type 1 diabetes development. Two species, Lactobacillus johnsonii and L. reuteri, were isolated from diabetes resistant rats. In this study diabetes prone rats were administered pure cultures of L. johnsonii or L. reuteri isolated from diabetes resistant rats to determine the effect on type 1 diabetes development.
Methodology/principal: Findings Results Rats administered L. johnsonii, but not L. reuteri, post-weaning developed type 1 diabetes at a protracted rate. Analysis of the intestinal ileum showed administration of L. johnsonii induced changes in the native microbiota, host mucosal proteins, and host oxidative stress response. A decreased oxidative intestinal environment was evidenced by decreased expression of several oxidative response proteins in the intestinal mucosa (Gpx1, GR, Cat). In L. johnsonii fed animals low levels of the pro-inflammatory cytokine IFNgamma were correlated with low levels of iNOS and high levels of Cox2. The administration of L. johnsonii also resulted in higher levels of the tight junction protein claudin.
Conclusions: It was determined that the administration of L. johnsonii isolated from BioBreeding diabetes resistant rats delays or inhibits the onset of type 1 diabetes in BioBreeding diabetes prone rats. Taken collectively, these data suggest that the gut and the gut microbiota are potential agents of influence in type 1 diabetes development. These data also support therapeutic efforts that seek to modify gut microbiota as a means to modulate development of this disorder.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Feeding design using BB-DP animals.
Arrows in black mark the time that feeding was started. The dashed line indicates daily feeding. The dashed box indicates the period in which rats developed T1D associated hyperglycemia.
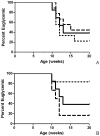
Figure 2. Kaplan-Meier plot depicting development of T1D in BB-DP rats.
Rats fed A) pre-weaning or B) post-weaning with L. johnsonii N6.2 (short dashed line), or L. reuteri TD1 (long dashed line) compared to the PBS fed control (solid line) N = 10 per group.
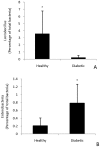
Figure 3. Quantification using real time qPCR of lactobacilli (A) and enterobacteria (B) from ileal mucosa.
The values are expressed as mean of the percentages from total bacteria determined from 5 ng of DNA. * indicates significant differences (P<0.05) between healthy and diabetic animals (N = 6 per group).
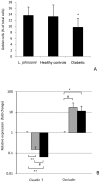
Figure 4. Effect of L. johnsonii administered post weaning on prevalence of goblet cells (A) and on mRNA levels of tight junction genes (B).
Hematoxylin and eosin stained slides of distal small intestine were examined for morphological changes. (A) Percentage of goblet cells in the distal small intestine by treatment group. (B) RT-qPCR analysis of the expression of tight junction genes. Relative amounts of claudin 1 and occludin were calculated by subtracting the internal control (β actin) and changes in expression levels were calculated relative to the value in the L. johnsonii fed group (expression = 1). Grey bars: Relative expression in the healthy control, Black bars: relative expression in the diabetic animals. The values are means +S.D. (N = 6); * P<0.05; # P<0.01; **P<0.0001
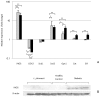
Figure 5. Assessment of the oxidative stress response in the host.
(A) RT-qPCR analysis of the expression of genes linked to the oxidative stress response in the host. Relative amounts of iNOS, Cox2, Sod1, Sod2, Gpx1, Cat, and GR were calculated by subtracting the internal control (β actin) and changes in expression levels were calculated relative to the value in L. johnsonii feed group (expression = 1). Grey bars: relative expression in the healthy control; Black bars: relative expression in the diabetic animals. The values are means +S.D. (N = 6); *P<0.05; °P<0.01, **P<0.0001. (B) Western blot analysis of iNOS levels. β-actin was used as internal control.
Figure 6. mRNA levels of the pro-inflammatory cytokine genes, IFNγ and TNFα linked to the oxidative stress response in the host.
Relative expression was calculated as previously described relative to the value in the L. johnsonii feed group (expression = 1). Relative expression in the L. johnsonii feed group (black bars), healthy control (dark grey bars), and diabetic animals (grey bars). The values are means +S.D. (N = 6); *P<0.05; # P<0.01.
References
- Yan F, Polk DB. Commensal bacteria in the gut: learning who our friends are. Curr Opin Gastroenterol. 2004;20:565–571. - PubMed
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. - PubMed
- Brugman S, Klatter FA, Visser JTJ, Wildeboer-Veloo ACM, Harmsen HJM, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. - PubMed
- Schwartz RF, Neu J, Schatz D, Atkinson MA, Wasserfall C. Comment on: Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2007;50:220–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous