Adult neurogenesis: integrating theories and separating functions - PubMed (original) (raw)
Review
Adult neurogenesis: integrating theories and separating functions
James B Aimone et al. Trends Cogn Sci. 2010 Jul.
Abstract
The continuous incorporation of new neurons in the dentate gyrus of the adult hippocampus raises exciting questions about memory and learning, and has inspired new computational models to understand the function of adult neurogenesis. These theoretical approaches suggest distinct roles for new neurons as they slowly integrate into the existing dentate gyrus network: immature adult-born neurons seem to function as pattern integrators of temporally adjacent events, thereby enhancing pattern separation for events separated in time; whereas maturing adult-born neurons possibly contribute to pattern separation by being more amenable to learning new information, leading to dedicated groups of granule cells that respond to experienced environments. We review these hypothesized functions and supporting empirical research and point to new directions for future theoretical efforts.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures
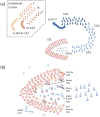
Figure 1. Hippocampal and DG anatomy
(a) Cartoon schematic of the hippocampus and its principal input, the entorhinal cortex. (b) Schematic of the dentate gyrus and the hilus. The major neuron classes of the DG are labeled.
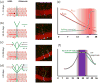
Figure 2. Maturation of adult-born neurons
(a) Seven- to 14-day-old neurons have limited dendritic arborization and receive only GABA inputs (red arrow), which are excitatory. Right: GFP labeled 10-day-old neuron. (b) Fourteen- to 21-day-old neurons begin to develop spines and receive glutamatergic inputs (blue arrows). GABA transitions from depolarizing to hyperpolarizing. Right: GFP-labeled 14-day-old neuron. (c) The 21- to 28-day-old neurons have significant spine formation and show increased plasticity of perforant path inputs; Right: GFP-labeled 21-day-old neuron. (d) By 56 days, neurons are comparable to embryonic-born GCs; Right: GFP-labeled 56-day-old neuron. (e) Cartoon schematic of maturation of basic neuronal electrophysiology properties. Period of potentially increased excitability is highlighted. F) Cartoon schematic of maturation of synaptic connectivity and plasticity. Period of potentially increased learning is highlighted. Image courtesy of Zhao et al., Journal of Neuroscience 2006[39].
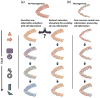
Figure 3. How DG and new neurons may affect pattern separation
Each panel represents how a series of temporally discrete events (left) would be encoded by the hippocampus. (a) Events encoded by the hippocampus without the DG would not be adequately separated, leading to possible clustering of memories based on content. (b) Events encoded by the DG without neurogenesis would be highly separated. (c) Events encoded by the DG with neurogenesis would also be highly separated but would potentially retain their temporal structure. This temporal integration would be provided by the heterogeneous mix of immature neurons.
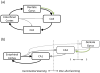
Figure 4. Alternative theories of neurogenesis depletion on the DG long-term
Each column represents how the DG would acquire information about events presented over extended time scales. (a) Without neurogenesis, the DG (and the rest of hippocampus) might continue to incorporate new information that, in effect, overwrites old information. Such a mechanism could lead to “catastrophic interference” in the network. (b) An alternative possibility is that, without neurogenesis, the DG network could quickly use its finite set of sparse codes for early events. All subsequent new memories would be encoded by utilizing already existing, and thus less optimal, sparse codes. (c) Most models of neurogenesis suggest that immature neurons are the most plastic in the network, allowing new information to be encoded by new neurons and leaving old information intact.
Figure 5. Role of DG and neurogenesis in hippocampal function
(a) According to theories based on the classic trisynaptic loop[23, 25, 88], the DG is the principal input to the hippocampus, positioning neurogenesis for a significant impact on memory formation. (b) An alternative possibility envisions that direct connections from EC to CA1 are sufficient for some hippocampal processing and loops through the CA3 and the DG are important under specific circumstances, such as one-shot learning[32, 89]. This positioning would suggest that DG modulates CA3 function and neurogenesis modulates this modulation.
Similar articles
- New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory?
Deng W, Aimone JB, Gage FH. Deng W, et al. Nat Rev Neurosci. 2010 May;11(5):339-50. doi: 10.1038/nrn2822. Epub 2010 Mar 31. Nat Rev Neurosci. 2010. PMID: 20354534 Free PMC article. Review. - Krüppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis.
Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Scobie KN, et al. J Neurosci. 2009 Aug 5;29(31):9875-87. doi: 10.1523/JNEUROSCI.2260-09.2009. J Neurosci. 2009. PMID: 19657039 Free PMC article. - Synapse formation on adult-born hippocampal neurons.
Toni N, Sultan S. Toni N, et al. Eur J Neurosci. 2011 Mar;33(6):1062-8. doi: 10.1111/j.1460-9568.2011.07604.x. Eur J Neurosci. 2011. PMID: 21395849 Review. - Long-lasting plasticity of hippocampal adult-born neurons.
Lemaire V, Tronel S, Montaron MF, Fabre A, Dugast E, Abrous DN. Lemaire V, et al. J Neurosci. 2012 Feb 29;32(9):3101-8. doi: 10.1523/JNEUROSCI.4731-11.2012. J Neurosci. 2012. PMID: 22378883 Free PMC article. - Adult neurogenesis in the intact and epileptic dentate gyrus.
Parent JM. Parent JM. Prog Brain Res. 2007;163:529-40. doi: 10.1016/S0079-6123(07)63028-3. Prog Brain Res. 2007. PMID: 17765736 Review.
Cited by
- Effect of adult-born immature granule cells on pattern separation in the hippocampal dentate gyrus.
Kim SY, Lim W. Kim SY, et al. Cogn Neurodyn. 2024 Aug;18(4):2077-2093. doi: 10.1007/s11571-023-09985-5. Epub 2023 Jul 1. Cogn Neurodyn. 2024. PMID: 39104672 - Could androgens maintain specific domains of mental health in aging men by preserving hippocampal neurogenesis?
Ransome MI. Ransome MI. Neural Regen Res. 2012 Oct 5;7(28):2227-39. doi: 10.3969/j.issn.1673-5374.2012.028.009. Neural Regen Res. 2012. PMID: 25538744 Free PMC article. Review. - Treadmill Exercise Reverses the Change of Dendritic Morphology and Activates BNDF-mTOR Signaling Pathway in the Hippocampus and Cerebral Cortex of Ovariectomized Mice.
Feng Y, Tian X, Zhang M, Lou S. Feng Y, et al. J Mol Neurosci. 2021 Sep;71(9):1849-1862. doi: 10.1007/s12031-021-01848-0. Epub 2021 May 26. J Mol Neurosci. 2021. PMID: 34041687 - Crosstalk among Epigenetic Pathways Regulates Neurogenesis.
Jobe EM, McQuate AL, Zhao X. Jobe EM, et al. Front Neurosci. 2012 May 8;6:59. doi: 10.3389/fnins.2012.00059. eCollection 2012. Front Neurosci. 2012. PMID: 22586361 Free PMC article. - Molecular layer perforant path-associated cells contribute to feed-forward inhibition in the adult dentate gyrus.
Li Y, Stam FJ, Aimone JB, Goulding M, Callaway EM, Gage FH. Li Y, et al. Proc Natl Acad Sci U S A. 2013 May 28;110(22):9106-11. doi: 10.1073/pnas.1306912110. Epub 2013 May 13. Proc Natl Acad Sci U S A. 2013. PMID: 23671081 Free PMC article.
References
- Zupanc GK. Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Brain Behav Evol. 2001;58:250–275. - PubMed
- Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. - PubMed
- Mouret A, et al. Centrifugal drive onto local inhibitory interneurons of the olfactory bulb. Ann N Y Acad Sci. 2009;1170:239–254. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources