Evolving role of MeCP2 in Rett syndrome and autism - PubMed (original) (raw)
Review
Evolving role of MeCP2 in Rett syndrome and autism
Janine M LaSalle et al. Epigenomics. 2009 Oct.
Abstract
Rett syndrome is an X-linked autism-spectrum disorder caused by mutations in MECP2, encoding methyl CpG-binding protein 2. Since the discovery of MECP2 mutations as the genetic cause of Rett syndrome, the understanding of MeCP2 function has evolved. Although MeCP2 was predicted to be a global transcriptional repressor of methylated promoters, large-scale combined epigenomic approaches of MeCP2 binding, methylation and gene expression have demonstrated that MeCP2 binds preferentially to intergenic and intronic regions, and sparsely methylated promoters of active genes. This review compares the evolution of thought within two ‘classic’ epigenetic mechanisms of parental imprinting and X chromosome inactivation to that of the MeCP2 field, and considers the future relevance of integrated epigenomic databases to understanding autism and Rett syndrome.
Keywords: MeCP2; Rett syndrome; X chromosome inactivation; autism; chromatin; epigenetic; imprinting; methylation.
Figures
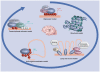
Figure 1. Evolving models of MeCP2 have come full circle
Models explaining the functional role of MeCP2 in the nucleus have evolved since the transcriptional repressor model of the 1990s. A structural model was later proposed based on studies showing MeCP2 associating with itself and DNA to form dense chromatin structures, consistent with its localization to nuclear heterochromatin. A ‘loop and recruit’ model incorporates a role for MeCP2 in chromatin loop structure, nuclear matrix binding, recruitment of RNA splicing and chromatin remodeling factors. An ‘active gene modulator’ model was supported by integrated epigenomic analyses showing MeCP2 binding to active gene promoters, but primarily to intronic and intergenic sites. And lastly, a transcriptional activator model was proposed based on the interaction of MeCP2 with the transcription factor CREB1. While the evolution of thought on MeCP2 has come full circle, the diversity of models is expected to reflect the diversity of roles for MeCP2 in vivo. The challenge for the field in the future is to determine which of the diverse MeCP2 roles are essential for the postnatal brain that lead to Rett syndrome when deficient. HDAC: Histone deacetylase.
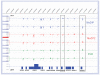
Figure 2. Combined epigenomic profiles at the HOXA gene locus exemplify the concordance of MeCP2 and gene activity with low methylation
Data taken from genome-wide promoter array analyses from Yasui et al. [10] is mapped at the HOXA gene locus on human chromosome 7, for which tissue-specific expression differences correlate with epigenetic markers. HOXA9 is poorly expressed in neurons and has high promoter methylation (MeDIP, blue), low MeCP2 binding (red), and low Pol2 association (green). In contrast, the active HOXA13 promoter shows low methylation but high MeCP2 and Pol2 binding. Overall, there was a striking correlation between MeCP2 and Pol2 chromatin immuno precipitation signals throughout the genome, exemplified at this locus of variable gene activity.
Similar articles
- Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3.
Samaco RC, Hogart A, LaSalle JM. Samaco RC, et al. Hum Mol Genet. 2005 Feb 15;14(4):483-92. doi: 10.1093/hmg/ddi045. Epub 2004 Dec 22. Hum Mol Genet. 2005. PMID: 15615769 Free PMC article. - Reciprocal co-regulation of EGR2 and MECP2 is disrupted in Rett syndrome and autism.
Swanberg SE, Nagarajan RP, Peddada S, Yasui DH, LaSalle JM. Swanberg SE, et al. Hum Mol Genet. 2009 Feb 1;18(3):525-34. doi: 10.1093/hmg/ddn380. Epub 2008 Nov 10. Hum Mol Genet. 2009. PMID: 19000991 Free PMC article. - Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes.
Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM. Yasui DH, et al. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19416-21. doi: 10.1073/pnas.0707442104. Epub 2007 Nov 27. Proc Natl Acad Sci U S A. 2007. PMID: 18042715 Free PMC article. - MeCP2 dysfunction in Rett syndrome and related disorders.
Moretti P, Zoghbi HY. Moretti P, et al. Curr Opin Genet Dev. 2006 Jun;16(3):276-81. doi: 10.1016/j.gde.2006.04.009. Epub 2006 May 2. Curr Opin Genet Dev. 2006. PMID: 16647848 Review. - The role of MeCP2 in brain development and neurodevelopmental disorders.
Gonzales ML, LaSalle JM. Gonzales ML, et al. Curr Psychiatry Rep. 2010 Apr;12(2):127-34. doi: 10.1007/s11920-010-0097-7. Curr Psychiatry Rep. 2010. PMID: 20425298 Free PMC article. Review.
Cited by
- Epigenomic strategies at the interface of genetic and environmental risk factors for autism.
LaSalle JM. LaSalle JM. J Hum Genet. 2013 Jul;58(7):396-401. doi: 10.1038/jhg.2013.49. Epub 2013 May 16. J Hum Genet. 2013. PMID: 23677056 Free PMC article. Review. - Traversing the epigenetic landscape: DNA methylation from retina to brain in development and disease.
Xu C, Fu X, Qin H, Yao K. Xu C, et al. Front Cell Neurosci. 2024 Nov 29;18:1499719. doi: 10.3389/fncel.2024.1499719. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39678047 Free PMC article. Review. - The landscape of DNA methylation amid a perfect storm of autism aetiologies.
Vogel Ciernia A, LaSalle J. Vogel Ciernia A, et al. Nat Rev Neurosci. 2016 Jul;17(7):411-23. doi: 10.1038/nrn.2016.41. Epub 2016 May 6. Nat Rev Neurosci. 2016. PMID: 27150399 Free PMC article. Review. - Chimeric MicroRNA-1291 Biosynthesized Efficiently in Escherichia coli Is Effective to Reduce Target Gene Expression in Human Carcinoma Cells and Improve Chemosensitivity.
Li MM, Addepalli B, Tu MJ, Chen QX, Wang WP, Limbach PA, LaSalle JM, Zeng S, Huang M, Yu AM. Li MM, et al. Drug Metab Dispos. 2015 Jul;43(7):1129-36. doi: 10.1124/dmd.115.064493. Epub 2015 May 1. Drug Metab Dispos. 2015. PMID: 25934574 Free PMC article. - Systems Genetics Reveals the Gene Regulatory Mechanisms of Arrb2 in the Development of Autism Spectrum Disorders.
Xia J, Bajpai AK, Liu Y, Yu L, Dong Y, Li F, Chen F, Lu L, Feng S. Xia J, et al. Genes (Basel). 2025 May 20;16(5):605. doi: 10.3390/genes16050605. Genes (Basel). 2025. PMID: 40428426 Free PMC article.
References
- Armstrong DD, Dunn K, Antalffy B. Decreased dendritic branching in frontal, motor and limbic cortex in Rett syndrome compared with trisomy 21. J. Neuropathol. Exp. Neurol. 1998;57(11):1013–1017. - PubMed
- Raymond GV, Bauman ML, Kemper TL. Hippocampus in autism: a Golgi analysis. Acta Neuropathol. 1996;91(1):117–119. - PubMed
- Belichenko PV, Oldfors A, Hagberg B, et al. 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport. 1994;5(12):1509–1513. - PubMed
- Pickett J, London E. The neuropathology of autism: a review. J. Neuropathol. Exp. Neurol. 2005;64(11):925–935. - PubMed
- Armstrong DD. Neuropathology of Rett syndrome. J. Child Neurol. 2005;20(9):747–753. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical