FT-ICR MS optimization for the analysis of intact proteins - PubMed (original) (raw)
FT-ICR MS optimization for the analysis of intact proteins
Aleksey V Tolmachev et al. Int J Mass Spectrom. 2009.
Abstract
Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometry (MS) remains the technique of choice for the analysis of intact proteins from complex biological systems, i.e. top-down proteomics. Recently, we have implemented a compensated open cylindrical ion trapping cell into a 12 T FT-ICR mass spectrometer. This new cell has previously demonstrated improved sensitivity, dynamic range, and mass measurement accuracy for the analysis of relatively small tryptic peptides. These improvements are due to the modified trapping potential of the cell which closely approximates the ideal harmonic trapping potential. Here, we report the instrument optimization for the analysis of large macro-molecular ions, such as proteins. Single transient mass spectra of multiply charged bovine ubiquitin ions with sub-ppm mass measurement accuracy, improved signal intensity, and increased dynamic range were obtained using this new cell with increased post-excitation cyclotron radii. The increased cyclotron radii correspond to increased ion kinetic energy and collisions between neutrals and ions with sufficient kinetic energy can exceed a threshold of single collision ion fragmentation. A transition then occurs from relatively long signal lifetimes at low excitation radii to potentially shorter lifetimes, defined by the average ion-neutral collision time. The proposed high energy ion loss mechanism is evaluated and compared with experimental results for bovine ubiquitin and serum albumin. We find that the analysis of large macro-molecules can be significantly improved by the further reduction of pressure in the ion trapping cell. This reduces the high energy ion losses and can enable increased sensitivity and mass measurement accuracy to be realized without compromising resolution. Further, these results appear to be generally applicable to FTMS, and it is expected that the high energy ion loss mechanism also applies to Orbitrap mass analyzers.
Figures
Figure 1
ESI mass spectrum of ubiquitin obtained using the compensated cell, with an excitation attenuation of 7 dB, from a single transient 1.3 s long, with resolution of ~230,000 and insert detail of the 11+ charge state.
Figure 2
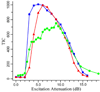
Total ion current (TIC) versus excitation power (plotted in terms of attenuation, dB) for the compensated cell configuration, spectra were acquired at ~1 × 10−10 Torr (squares), open cell configuration, ~1 × 10−10 Torr (triangles), and compensated cell configuration at an increased pressure of ~3 × 10−10 Torr (circles).
Figure 3
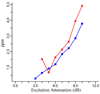
Mass measurement error, plotted as ppm, versus excitation power, plotted in terms of attenuation dB, for the compensated cell configuration (squares) and open cell configuration (triangles). The internal calibration using 6 charge states of ubiquitine was used for the mass measurement error calculation.
Figure 4
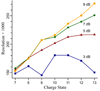
Resolution of ubiquitin 7 to 13+ obtained at excitation attenuations of 9 dB (circles), 7 dB (diamonds), 5 dB (triangles), and 3 dB (squares) corresponding to approximately 0.4, 0.5, 0.6, and 0.75 of the maximum cell radius, Rmax, of ~3.0 cm.
Figure 5
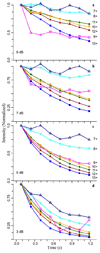
Transient decay curves showing the intensity of the most abundant isotope peak for ubiquitin 7 to 13+ charge states versus time obtained by Fourier-transforming the full 1 M word transient in eight 128 k word segments. Each transformed segment measures the ion signal obtained at a certain time point in the transient. The observed segment intensities were normalized to the intensity of the first segment for each charge state. Plots are shown for mass spectra obtained at excitation attenuations of a) 9 dB, b) 7 dB, c) 5 dB and d) 3 dB for ubiquitin 7+ (open triangles), 8+ (open circles), 9+ (open squares), 10+ (solid circles), 11+ (diamonds), 12+ (solid triangles), and 13+ (solid squares).
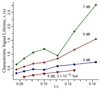
Figure 6
The characteristic signal lifetime, τ, for ubiquitin charges states 7 to 13+ (plotted as 1/z) calculated by fitting the transient decay curves (see Figure 5) observed at 7 dB (diamonds), 5 dB (triangles), and 3 dB (squares) to an exponential function. Results from a pressure of ~1 (triangles pointing up) and 3 (triangles pointing down) × 10−10 Torr are shown for an excitation attenuation of 5 dB. Pressure for all others was ~1 × 10−10 Torr.
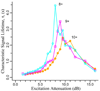
Figure 7
The characteristic signal lifetime, τ, at different excitation power levels (in terms of attenuation dB) for ubiquitin 8+ (open circles), 9+ (squares), 10+ (solid circles), at a pressure 3 × 10−10 Torr.
Figure 8
LC-MS mass spectra of bovine serum albumin (BSA) obtained by summing 50 scans of the BSA LC peak. Several isoforms with various adducts were observed and the base peak of the 63+ cluster is shown in the insert.
Similar articles
- High-resolution Fourier transform ion cyclotron resonance mass spectrometry with increased throughput for biomolecular analysis.
Nagornov KO, Gorshkov MV, Kozhinov AN, Tsybin YO. Nagornov KO, et al. Anal Chem. 2014 Sep 16;86(18):9020-8. doi: 10.1021/ac501579h. Epub 2014 Sep 3. Anal Chem. 2014. PMID: 25140615 - Narrow Aperture Detection Electrodes ICR Cell with Quadrupolar Ion Detection for FT-ICR MS at the Cyclotron Frequency.
Nagornov KO, Kozhinov AN, Nicol E, Tsybin OY, Touboul D, Brunelle A, Tsybin YO. Nagornov KO, et al. J Am Soc Mass Spectrom. 2020 Nov 4;31(11):2258-2269. doi: 10.1021/jasms.0c00221. Epub 2020 Oct 5. J Am Soc Mass Spectrom. 2020. PMID: 32966078 - Collisional activation of peptide ions in FT-ICR mass spectrometry.
Laskin J, Futrell JH. Laskin J, et al. Mass Spectrom Rev. 2003 May-Jun;22(3):158-81. doi: 10.1002/mas.10041. Mass Spectrom Rev. 2003. PMID: 12838543 Review. - Activation of large ions in FT-ICR mass spectrometry.
Laskin J, Futrell JH. Laskin J, et al. Mass Spectrom Rev. 2005 Mar-Apr;24(2):135-67. doi: 10.1002/mas.20012. Mass Spectrom Rev. 2005. PMID: 15389858 Review. - Trapped-ion cell with improved DC potential harmonicity for FT-ICR MS.
Tolmachev AV, Robinson EW, Wu S, Kang H, Lourette NM, Pasa-Tolić L, Smith RD. Tolmachev AV, et al. J Am Soc Mass Spectrom. 2008 Apr;19(4):586-97. doi: 10.1016/j.jasms.2008.01.006. Epub 2008 Jan 31. J Am Soc Mass Spectrom. 2008. PMID: 18296061 Free PMC article.
Cited by
- 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer: A National Resource for Ultrahigh Resolution Mass Analysis.
Hendrickson CL, Quinn JP, Kaiser NK, Smith DF, Blakney GT, Chen T, Marshall AG, Weisbrod CR, Beu SC. Hendrickson CL, et al. J Am Soc Mass Spectrom. 2015 Sep;26(9):1626-32. doi: 10.1007/s13361-015-1182-2. Epub 2015 Jun 20. J Am Soc Mass Spectrom. 2015. PMID: 26091892 - Nano-LC FTICR tandem mass spectrometry for top-down proteomics: routine baseline unit mass resolution of whole cell lysate proteins up to 72 kDa.
Tipton JD, Tran JC, Catherman AD, Ahlf DR, Durbin KR, Lee JE, Kellie JF, Kelleher NL, Hendrickson CL, Marshall AG. Tipton JD, et al. Anal Chem. 2012 Mar 6;84(5):2111-7. doi: 10.1021/ac202651v. Epub 2012 Feb 22. Anal Chem. 2012. PMID: 22356091 Free PMC article. - Transformative effects of higher magnetic field in Fourier transform ion cyclotron resonance mass spectrometry.
Karabacak NM, Easterling ML, Agar NY, Agar JN. Karabacak NM, et al. J Am Soc Mass Spectrom. 2010 Jul;21(7):1218-22. doi: 10.1016/j.jasms.2010.03.033. Epub 2010 Mar 31. J Am Soc Mass Spectrom. 2010. PMID: 20444622 Free PMC article. - Mass spectrometry-based proteomics: existing capabilities and future directions.
Angel TE, Aryal UK, Hengel SM, Baker ES, Kelly RT, Robinson EW, Smith RD. Angel TE, et al. Chem Soc Rev. 2012 May 21;41(10):3912-28. doi: 10.1039/c2cs15331a. Epub 2012 Apr 13. Chem Soc Rev. 2012. PMID: 22498958 Free PMC article. Review.
References
- Guan SH, Marshall AG, Scheppele SE. Resolution and chemical formula identification of aromatic hydrocarbons and aromatic compounds containing sulfur, nitrogen, or oxygen in petroleum distillates and refinery streams. Anal. Chem. 1996;68:46–71. - PubMed
- Wu ZG, Rodgers RP, Marshall AG. ESI FT-ICR mass spectral analysis of coal liquefaction products. Fuel. 2005;84:1790–1797.
- Wu ZG, Rodgers RP, Marshall AG. Characterization of vegetable oils: Detailed compositional fingerprints derived from electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. J. Agric. Food. Chem. 2004;52:5322–5328. - PubMed
- Takats Z, Kobliha V, Sevcik K, Novak P, Kruppa G, Lemr K, Havlicek V. Characterization of DESI-FTICR mass spectrometry - from ECD to accurate mass tissue analysis. J. Mass Spectrom. 2008;43:196–203. - PubMed
- Brown SC, Kruppa G, Dasseux JL. Metabolomics applications of FT-ICR mass spectrometry. Mass Spectrom. Rev. 2005;24:223–231. - PubMed
LinkOut - more resources
Full Text Sources