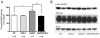Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer's disease - PubMed (original) (raw)
Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer's disease
Cynthia A Massaad et al. PLoS One. 2010.
Abstract
Background: Alzheimer's disease (AD) is a neurodegenerative disease characterized by the progressive decline in cognitive functions and the deposition of aggregated amyloid beta (Abeta) into senile plaques and the protein tau into tangles. In addition, a general state of oxidation has long been known to be a major hallmark of the disease. What is not known however, are the mechanisms by which oxidative stress contributes to the pathology of AD.
Methodology/principal findings: In the current study, we used a mouse model of AD and genetically boosted its ability to quench free radicals of specific mitochondrial origin. We found that such manipulation conferred to the AD mice protection against vascular as well as neuronal deficits that typically affect them. We also found that the vascular deficits are improved via antioxidant modulation of the endothelial nitric oxide synthase, an enzyme primarily responsible for the production of nitric oxide, while neuronal deficits are improved via modulation of the phosphorylation status of the protein tau, which is a neuronal cytoskeletal stabilizer.
Conclusions/significance: These findings directly link free radicals of specific mitochondrial origin to AD-associated vascular and neuronal pathology.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
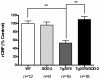
Figure 1. SOD-2 overexpression improves the regional cerebral blood flow (rCBF) deficits displayed in Tg2576 mice.
A) The graph represents the regional cerebral blood flow (rCBF) levels in 12 to 16 months old WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice as measured by MRI (ASL). Significance was assessed by a one-way ANOVA with Dunnett's post-test for multiple comparisons. ** p<0.01.
Figure 2. SOD-2 overexpression prevents increases in phospho-eNOS-ser1177 in Tg2576 mice.
A) Graph represents quantification of the levels of phospho-eNOS-ser1177 normalized to total eNOS from 12 to 16 months old cortical homogenates of WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice. Significance was assessed by one way ANOVA with Dunnett's post-test for multiple comparisons. *p<0.05. B) Representative Western blot of phospho-eNOS-Ser1177, total eNOS and β-actin from cortical homogenates of 12 to 16 months old WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice.
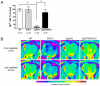
Figure 3. SOD-2 overexpression improves the axonal transport deficits displayed by Tg2576 mice.
A) The graph represents the rates on axonal transport as measured in vivo by MEMRI in 12 to 16 months old WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice. Significance was assessed by a one-way ANOVA with Dunnett's post-test for multiple comparisons. ** p<0.01. B) Representative MR images with a pseudo-color overlay showing manganese accumulation in the region of interest (indicated by an arrow) in the olfactory bulb of 12 to 16 months old WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice. Top row images represent the first repetition of the scan, where axonal transport of manganese has not started yet. Bottom row images represent the last repetition of the scan, where most of the manganese has already accumulated in the region of interest. Note that for the Tg2576 mice, no manganese accumulated in the region of interest which is indicative of deficient axonal transport. In the Tg2576/SOD-2 mice, accumulation of manganese is intact, indicating recovered axonal transport. The two images from each animal are on the same brightness scale.
Figure 4. SOD-2 overexpression prevents increases in phospho-tau-ser262 in Tg2576 mice.
A) Graph represents quantification of the levels of phospho-tau-ser262 normalized to total tau from 12 to 16 months old olfactory bulb homogenates of WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice. Significance was assessed by one way ANOVA with Dunnett's post-test for multiple comparisons. *p<0.05; **p<0.01. B) Representative Western blot of phospho-tau-Ser262, total tau and β-actin from olfactory bulb homogenates of 12 to 16 months old WT, SOD-2, Tg2576 and Tg2576/SOD-2 mice.
Similar articles
- Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer's disease.
Massaad CA, Washington TM, Pautler RG, Klann E. Massaad CA, et al. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13576-81. doi: 10.1073/pnas.0902714106. Epub 2009 Aug 3. Proc Natl Acad Sci U S A. 2009. PMID: 19666610 Free PMC article. - Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer's disease.
Chiasseu M, Alarcon-Martinez L, Belforte N, Quintero H, Dotigny F, Destroismaisons L, Vande Velde C, Panayi F, Louis C, Di Polo A. Chiasseu M, et al. Mol Neurodegener. 2017 Aug 3;12(1):58. doi: 10.1186/s13024-017-0199-3. Mol Neurodegener. 2017. PMID: 28774322 Free PMC article. - Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.
Terwel D, Dewachter I, Van Leuven F. Terwel D, et al. Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review. - Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.
Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribé E, Dalfó E, Avila J. Ferrer I, et al. Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
Cited by
- Pharmocologic treatment with histone deacetylase 6 inhibitor (ACY-738) recovers Alzheimer's disease phenotype in amyloid precursor protein/presenilin 1 (APP/PS1) mice.
Majid T, Griffin D, Criss Z 2nd, Jarpe M, Pautler RG. Majid T, et al. Alzheimers Dement (N Y). 2015 Oct 11;1(3):170-181. doi: 10.1016/j.trci.2015.08.001. eCollection 2015 Nov. Alzheimers Dement (N Y). 2015. PMID: 29854936 Free PMC article. - Targeting NOX enzymes in the central nervous system: therapeutic opportunities.
Sorce S, Krause KH, Jaquet V. Sorce S, et al. Cell Mol Life Sci. 2012 Jul;69(14):2387-407. doi: 10.1007/s00018-012-1014-5. Epub 2012 May 30. Cell Mol Life Sci. 2012. PMID: 22643836 Free PMC article. Review. - Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer's Disease.
Parodi-Rullán R, Sone JY, Fossati S. Parodi-Rullán R, et al. J Alzheimers Dis. 2019;72(4):1019-1039. doi: 10.3233/JAD-190357. J Alzheimers Dis. 2019. PMID: 31306129 Free PMC article. Review. - Mitochondrial abnormalities in Alzheimer's disease: possible targets for therapeutic intervention.
Silva DF, Selfridge JE, Lu J, E L, Cardoso SM, Swerdlow RH. Silva DF, et al. Adv Pharmacol. 2012;64:83-126. doi: 10.1016/B978-0-12-394816-8.00003-9. Adv Pharmacol. 2012. PMID: 22840745 Free PMC article. Review. - Improvements in a Mouse Model of Alzheimer's Disease Through SOD2 Overexpression are Due to Functional and Not Structural Alterations.
Bitner BR, Perez-Torres CJ, Hu L, Inoue T, Pautler RG. Bitner BR, et al. Magn Reson Insights. 2012 Mar 29;5:1-6. doi: 10.4137/MRI.S9352. Magn Reson Insights. 2012. PMID: 22639527 Free PMC article.
References
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
- Behl C. Oxidative stress in Alzheimer's disease: implications for prevention and therapy. Subcell Biochem. 2005;38:65–78. - PubMed
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. - PubMed
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG029977/AG/NIA NIH HHS/United States
- R01 NS047384/NS/NINDS NIH HHS/United States
- NS047384/NS/NINDS NIH HHS/United States
- R37 NS034007/NS/NINDS NIH HHS/United States
- R29 NS034007/NS/NINDS NIH HHS/United States
- AG029977/AG/NIA NIH HHS/United States
- R01 NS034007/NS/NINDS NIH HHS/United States
- T32 HL007676/HL/NHLBI NIH HHS/United States
- NS034007/NS/NINDS NIH HHS/United States
- T32HL07676/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases