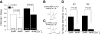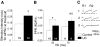Presynaptic inhibitory terminals are functionally abnormal in a rat model of posttraumatic epilepsy - PubMed (original) (raw)
Presynaptic inhibitory terminals are functionally abnormal in a rat model of posttraumatic epilepsy
Leonardo C Faria et al. J Neurophysiol. 2010 Jul.
Abstract
Partially isolated "undercut" neocortex with intact pial circulation is a well-established model of posttraumatic epileptogenesis. Results of previous experiments showed a decreased frequency of miniature inhibitory postsynaptic currents (mIPSCs) in layer V pyramidal (Pyr) neurons of undercuts. We further examined possible functional abnormalities in GABAergic inhibition in rat epileptogenic neocortical slices in vitro by recording whole cell monosynaptic IPSCs in layer V Pyr cells and fast-spiking (FS) GABAergic interneurons using a paired pulse paradigm. Compared with controls, IPSCs in Pyr neurons of injured slices showed increased threshold and decreased peak amplitude at threshold, decreased input/output slopes, increased failure rates, and a shift from paired pulse depression toward paired pulse facilitation (increased paired pulse ratio or PPR). Increasing [Ca(2+)](o) from 2 to 4 mM partially reversed these abnormalities in Pyr cells of the epileptogenic tissue. IPSCs onto FS cells also had an increased PPR and failures. Blockade of GABA(B) receptors did not affect the paired results. These findings suggest that there are functional alterations in GABAergic presynaptic terminals onto both Pyr and FS cells in this model of posttraumatic epileptogenesis.
Figures
Fig. 1.
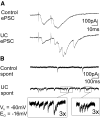
Epileptform activity in injured layer V pyramidal (Pyr) neurons. A: representative postsynaptic currents (PSCs) evoked by pairs of 1.5 T stimuli in a neuron of control (top) and undercut (UC) slice (bottom). In UC cell, the 2nd stimulus evokes a burst of PSCs. B: spontaneous PSCs in neurons from control (top) and UC slices (bottom). Bursts of PSCs occur in UC but not in control cell. Segments in boxes shown at 3× gain and sweep speed. _V_h: −60 mV; _E_Cl: −16 mV in A and B so that both excitatory and inhibitory PSCs (EPSCs and IPSCs) likely contribute to the spontaneous and evoked PSCs.
Fig. 2.
Threshold and mean slope of input output curves for evoked IPSCs (eIPSCs) in Pyr neurons of control and UC slices. A: the stimulus intensity required to evoke IPSCs at threshold was higher in UCs than in control group. Pulse width: 100 μs. B: mean slope in control (▵) is steeper than in UCs (▲) _R_2 control = 0.77 ± 0.04, n = 28; _R_2 UC = 0.61 ± 0.07, n = 22; P < 0.05. Inset: representative eIPSCs from UC (top) and control (bottom).
Fig. 3.
Increased paired pulse ratio (PPR) in injured Pyr neurons of UC rats. A: plot of PPR vs. interstimulus interval for control and UC neurons. At interspike intervals (ISIs) of 50 and 75 ms, but not 20 ms, the PPR for eIPSCs in layer V Pyr neurons of UC slices was significantly different from control and shifted toward facilitation (values given in Table 2). Inset: representative responses to paired stimuli (ISI = 50 ms) from control (top) and UC neurons (bottom), obtained at V_h = −60 mV. - - -, peak amplitude of R1 here and in Fig. 7_C. B: normalized peak amplitudes for evoked IPSCs decrease progressively during trains of 8 stimuli. Mean slopes for normalized peak amplitudes are steeper in control cells (P < 0.05). Inset: representative responses for a control (bottom) and UC neuron (top). C: stimulus intensity affects peak amplitude but not PPR. Peak amplitude (left) increased when stimulus intensity was increased from 1.5 to 2.5 –3× threshold in UCs (n = 7; P < 0.005), without a change in PPR (right). Inset: pairs of representative eIPSCs show increased peak amplitude for 2.5T vs. 1.5T stimuli. Calibration: 100 pA, 50 ms.
Fig. 4.
Relationship between amplitudes of R1 and R2 in control and UC layer V Pyr cells. A: plot of peak amplitude of the 1st (R1) vs. the 2nd response (R2) for 15 pairs of eIPSCs (ISI 50 ms) recorded in a representative control Pyr cell. Line: linear regression, _R_2 = 0.15. B: mean group values for slopes of R1 vs. R2 amplitudes in control and UC. Control _R_2 = 0.16 ± 0.03; UC _R_2 = 0.21 ± 0.04; P > 0.05). Numbers of neurons shown in each column.
Fig. 5.
Failure rate for eIPSCs in layer V Pyr neurons from UCs is increased vs. controls. A: representative responses to 1.5T stimuli showing failures of R1 or R2 that often occurred in the UC group (middle and bottom) but not in control (top). B: at ISI = 50 ms, there were no failures of R1 and 0.3 ± 0.3% failures of R2 in control cells. In contrast, failures occurred in 5.2 ± 1.2 and 5.5 ± 1.3% of R1 and R2, respectively, in UCs.
Fig. 6.
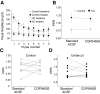
Effects of increased [Ca2+]o on PPR and failure rate in Pyr neurons. A: increasing [Ca2+] in artificial cerebrospinal fluid (ACSF) from 2 to 4 mM decreased PPR in control (□, left) and shifted values toward control in UCs (■; right). B and C: representative eIPSC responses to paired stimuli (ISI 50 ms) in 4 mM [Ca2+] in control (B) and UC cells (C). PPRs of both representative samples are similar to the mean group values in 4 mM [Ca2+] in graph of A. D: incubation in 4 mM [Ca2+] markedly decreased failures of R1 and R2 in Pyr cells from UC rats. The effect of high calcium on R1 and R2 was similar. Calibration in B and C: 100 pA, 40 ms.
Fig. 7.
GABAB receptor blockade does not affect amplitude or PPR of eIPSCs in control or injured layer V Pyr neurons. A: slopes of mean peak amplitudes vs. pulse number in standard ACSF (□, ○) and after GABAB receptor blockade (■, ●). B: PPR (ISI = 50 ms) recorded before and after GABAB receptor blockade was not affected in either control (n = 10) or UC (n = 9) group (control PPR = 0.77 ± 0.08; 10 μM CGP54626 PPR = 0.79 ± 0.06; P > 0.05). C and D: control (C) and UC group (D) variability in PPR during perfusion of standard ACSF and after bath perfusion of ACSF containing 10 μM CGP54626. Lines connect PPR values for single neurons before and after GABAB receptor blockade.
Fig. 8.
Inhibitory innervation of fast-spiking (FS) cells is altered in UCs. A: threshold for evoking IPSCs onto FS cells of UC is increased compared with control. Stimulus pulse duration was set at 100 μs and intensity increased until ∼50% of stimuli evoked an IPSC. B: PPR is increased in FS cells of UC at ISI = 50 ms. A nonsignificant shift in PPR toward facilitation was present at ISI = 75 ms. C: representative IPSCs evoked by paired stimuli show PPD in control neuron (C1) and decreased PPD in a cell from UC (C2; ISI = 50 ms, 1.5T, _V_h = −60 mV, _E_Cl = −16 mV). Failures persisted in UCs when stimulus width was increased to 1.5T (C3). Numbers in bars of A and B: total number of FS cells recorded. - - - in C, 1 and 2, mark peak of R1 (C1) and of R2 (C2).
Similar articles
- Structural alterations in fast-spiking GABAergic interneurons in a model of posttraumatic neocortical epileptogenesis.
Gu F, Parada I, Shen F, Li J, Bacci A, Graber K, Taghavi RM, Scalise K, Schwartzkroin P, Wenzel J, Prince DA. Gu F, et al. Neurobiol Dis. 2017 Dec;108:100-114. doi: 10.1016/j.nbd.2017.08.008. Epub 2017 Aug 18. Neurobiol Dis. 2017. PMID: 28823934 Free PMC article. - Functional alterations in GABAergic fast-spiking interneurons in chronically injured epileptogenic neocortex.
Ma Y, Prince DA. Ma Y, et al. Neurobiol Dis. 2012 Jul;47(1):102-13. doi: 10.1016/j.nbd.2012.03.027. Epub 2012 Mar 29. Neurobiol Dis. 2012. PMID: 22484482 Free PMC article. - Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex.
Li H, Prince DA. Li H, et al. J Neurophysiol. 2002 Jul;88(1):2-12. doi: 10.1152/jn.00507.2001. J Neurophysiol. 2002. PMID: 12091528 - Fast-spiking cell to pyramidal cell connections are the most sensitive to propofol-induced facilitation of GABAergic currents in rat insular cortex.
Koyanagi Y, Oi Y, Yamamoto K, Koshikawa N, Kobayashi M. Koyanagi Y, et al. Anesthesiology. 2014 Jul;121(1):68-78. doi: 10.1097/ALN.0000000000000183. Anesthesiology. 2014. PMID: 24577288 - Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis.
Prince DA, Parada I, Scalise K, Graber K, Jin X, Shen F. Prince DA, et al. Epilepsia. 2009 Feb;50 Suppl 2(Suppl 2):30-40. doi: 10.1111/j.1528-1167.2008.02008.x. Epilepsia. 2009. PMID: 19187292 Free PMC article. Review.
Cited by
- Synaptic Integration in CA1 Pyramidal Neurons Is Intact despite Deficits in GABAergic Transmission in the Scn1a Haploinsufficiency Mouse Model of Dravet Syndrome.
Chancey JH, Howard MA. Chancey JH, et al. eNeuro. 2022 May 17;9(3):ENEURO.0080-22.2022. doi: 10.1523/ENEURO.0080-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35523580 Free PMC article. - Targets for preventing epilepsy following cortical injury.
Li H, McDonald W, Parada I, Faria L, Graber K, Takahashi DK, Ma Y, Prince D. Li H, et al. Neurosci Lett. 2011 Jun 27;497(3):172-6. doi: 10.1016/j.neulet.2011.02.042. Epub 2011 Feb 24. Neurosci Lett. 2011. PMID: 21354270 Free PMC article. Review. - Prolonged prophylactic effects of gabapentin on status epilepticus-induced neocortical injury.
Perez-Ramirez MB, Gu F, Prince DA. Perez-Ramirez MB, et al. Neurobiol Dis. 2020 Aug;142:104949. doi: 10.1016/j.nbd.2020.104949. Epub 2020 May 19. Neurobiol Dis. 2020. PMID: 32442680 Free PMC article. - Partial Activation of TrkB Receptors Corrects Interneuronal Calcium Channel Dysfunction and Reduces Epileptogenic Activity in Neocortex following Injury.
Gu F, Parada I, Yang T, Longo FM, Prince DA. Gu F, et al. Cereb Cortex. 2020 Jul 30;30(9):5180-5189. doi: 10.1093/cercor/bhz254. Cereb Cortex. 2020. PMID: 32488246 Free PMC article. - Interneuronal calcium channel abnormalities in posttraumatic epileptogenic neocortex.
Faria LC, Parada I, Prince DA. Faria LC, et al. Neurobiol Dis. 2012 Feb;45(2):821-8. doi: 10.1016/j.nbd.2011.11.006. Epub 2011 Dec 7. Neurobiol Dis. 2012. PMID: 22172650 Free PMC article.
References
- Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to development of spontaneous recurrent seizures in the rat lithium-pilocarpine model of temporal lobe epilepsy. Hippocampus 11: 452–468, 2001 - PubMed
- Asprodini EK, Rainnie DG, Shinnick-Gallagher P. Epileptogenesis reduces the sensitivity of presynaptic gamma- aminobutyric acid B receptors on glutamatergic afferents in the amygdala. J Pharmacol Exp Ther 262: 1011–1021, 1992 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous