An allosteric mechanism to displace nuclear export cargo from CRM1 and RanGTP by RanBP1 - PubMed (original) (raw)
An allosteric mechanism to displace nuclear export cargo from CRM1 and RanGTP by RanBP1
Masako Koyama et al. EMBO J. 2010.
Abstract
The karyopherin CRM1 mediates nuclear export of proteins and ribonucleoproteins bearing a leucine-rich nuclear export signal (NES). To elucidate the precise mechanism by which NES-cargos are dissociated from CRM1 in the cytoplasm, which is important for transport directionality, we determined a 2.0-A resolution crystal structure of yeast CRM1:RanBP1:RanGTP complex, an intermediate in the disassembly of the CRM1 nuclear export complex. The structure shows that on association of Ran-binding domain (RanBD) of RanBP1 with CRM1:NES-cargo:RanGTP complex, RanBD and the C-terminal acidic tail of Ran induce a large movement of the intra-HEAT9 loop of CRM1. The loop moves to the CRM1 inner surface immediately behind the NES-binding site and causes conformational rearrangements in HEAT repeats 11 and 12 so that the hydrophobic NES-binding cleft on the CRM1 outer surface closes, squeezing out the NES-cargo. This allosteric mechanism accelerates dissociation of NES by over two orders of magnitude. Structure-based mutagenesis indicated that the HEAT9 loop also functions as an allosteric autoinhibitor to stabilize CRM1 in a conformation that is unable to bind NES-cargo in the absence of RanGTP.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
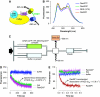
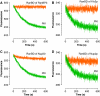
NES dissociation kinetics measured by a FRET-based assay. (A) Design of FRET constructs to detect the specific binding of Leu-rich NES to CRM1 and RanGTP. CFP was fused to the C-terminus of CRM1, whereas YFP was fused to the N-terminus of an NES (PKI residues 38–47; LALKLAGLDI; a representative Leu-rich NES). (B) Steady-state fluorescence spectra. Blue: 0.2 μM yeast CRM1-CFP, 1.0 μM YFP-NES, 3.0 μM yeast RanGTP. Green: 0.2 μM yeast CRM1-CFP, 1.0 μM YFP-NES, 3.0 μM yeast RanGDP. Magenta: 0.2 μM yeast CRM1-CFP, 1.0 μM YFP-NES (I47A), 3.0 μM yeast RanGTP. Orange: 0.2 μM yeast CRM1-CFP, 1.0 μM YFP-NES, 3.0 μM yeast RanGTP, 4.0 μM yeast RanBP1. (C) Setup of stopped-flow experiments to measure the time-dependent decrease in the FRET signal after rapid mixing. (D, E) 0.4 μM yeast CRM1-CFP, 2.0 μM YFP-NES (PKI residues 38–47; LALKLAGLDI) and 6.0 μM yeast RanGTP were preincubated, rapidly mixed with equal volume of either buffer alone (blue) or 80 μM PKI (green) or 8 μM yeast RanGAP (magenta) or 8 μM yeast RanBP1 (red), and then the time-dependent change in the CFP-YFP FRET signal was recorded. The green trace represents the spontaneous dissociation of NES from CRM1 and RanGTP (_k_off ∼0.0073/s). The red trace represents the RanBP1-accelerated release of NES from CRM1 and RanGTP (fitted exponential curve is superimposed on the stopped-flow trace; _k_off ∼15.6/s). Yeast RanBP1 dramatically increased the NES off-rate by ∼2000-fold. These data provided direct evidence that RanBP1 uses an active-displacement mechanism to dissociate NES-cargo from CRM1 and RanGTP.
Figure 2
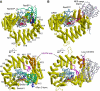
Overview of crystal structures. (A) Yeast CRM1:RanBP1:RanGTP complex (this study; PDB accession code: 3M1I). (B) CRM1:Spn1:RanGTP complex (Monecke et al, 2009; PDB accession code: 3GJX). The HEAT repeats of CRM1 are labelled H1–H21. CRM1 is coloured in yellow, except that HEAT9 loop (that shows large movement between the two structures) and HEAT repeats 11–12 (that constitute the NES-binding site) are highlighted in magenta and orange, respectively. Ran is coloured in cyan (switch I, switch II and the C-terminal tail are highlighted in pink, grey and blue, respectively; GTP is shown as space-filling spheres). RanBP1 and Spn1 are coloured in green and light grey, respectively. Leu-rich NES at the N-terminus of Spn1 (residues 1–16), highlighted in purple, binds to the outer surface of CRM1, away from Ran, whereas RanBP1 makes intimate contacts with RanGTP and binds to a site that is distinct from the NES-binding site. The structures suggest that RanBP1, together with the C-terminal tail of Ran, induces a large movement of HEAT9 loop that in turn causes conformational rearrangements in HEAT repeats 11–12 so that the hydrophobic NES-binding cleft on the CRM1 outer surface closes, squeezing out the NES-cargo (see Figures 3 and 4 and Supplementary Movies S1–S3 for details).
Figure 3
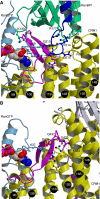
RanBP1 induces movement of HEAT9 loop by recruiting the C-terminal tail of Ran. Close-up view into the region where the C-terminal tail of Ran clashes with HEAT9 loop is shown. (A) Yeast CRM1:RanBP1:RanGTP complex. (B) CRM1:Spn1:RanGTP complex (CRM1 is mouse, whereas Spn1 and Ran are human) (Monecke et al, 2009). Colouring is the same as in Figure 2. Dashed lines in light blue indicate hydrogen bonds or salt bridges. GTP is shown as space-filling spheres. Residue numbering differs between species. Comparison of the two structures suggests that the C-terminus of Ran (blue in (A)) would clash with HEAT9 loop (magenta) and displace it on RanBP1 binding.
Figure 4
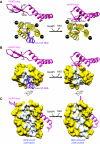
Structural rearrangements of the NES-binding cleft associated with RanBP1 binding and NES release. This figure illustrates how RanBP1-induced movement of HEAT9 loop forces CRM1 into a conformation that is incompatible with NES binding. Only HEAT repeats 9, 11 and 12 of CRM1 are shown for clarity. Left, CRM1:Spn1:RanGTP complex (Monecke et al, 2009); right, yeast CRM1:RanBP1:RanGTP complex. (A) Top view. HEAT9, magenta; HEAT11 and HEAT12, yellow; Leu-rich NES of Spn1, purple. Hydrophobic interaction between highly conserved hydrophobic residues of HEAT9 loop (Val441, Leu442, Val443, Ile451 of yeast CRM1, which correspond to Val430, Leu431, Val432, Val440 of mouse CRM1, respectively) and the inner surface of HEAT repeats 11 and 12 appears to induce movements of Met594, Met556, Phe583 of yeast CRM1 (Met583, Met545, Phe572 of mouse CRM1, respectively), leading to closure of the hydrophobic NES-binding cleft between outer helices 11A and 12A. (B) Same view as (A), but HEAT repeats 11 and 12 are shown in surface representation (the residues that directly interact with Leu-rich NES are white, whereas the other residues are yellow). (C) Side view. Colouring is the same as in (B). Only the side chains of Leu-rich NES are shown for clarity. Supplementary Movies S1–S3 show animation of the structural changes; Supplementary Figure S5 shows superposition of the two structures.
Figure 5
Structure-based mutants verified the allosteric mechanism of NES release. (A) A pulldown assay showed that the C-terminal tail of Ran is required for RanBP1-mediated dissociation of NES from CRM1 and RanGTP. Immobilized GST-PKI (60 μg) was incubated with CRM1 (68 μg) and RanGTP (48 μg), unbound CRM1 and Ran washed away and then the beads were incubated without (lanes 1 and 3) or with (lanes 2 and 4) RanBP1 (130 μg). Bound and unbound fractions were analysed by SDS–PAGE. Lanes 1 and 2, full-length Ran; lanes 3 and 4, a C-terminal deletion mutant of Ran (residues 1–211). As the band of RanBP1 overlapped with that of GST-PKI, RanBP1 in the bound fraction was detected by western blotting; the other proteins were detected by Coomassie staining. (B–D) Mutational analysis demonstrated that the conserved hydrophobic residues in HEAT9 loop are important for loading and unloading of NES-cargo. (B) Pulldown assays to analyse the effects of mutating hydrophobic residues of HEAT9 loop on the binding of yeast CRM1 to PKI (NES-cargo) in the absence (lanes 1–4) or presence (lanes 5–8) of RanGTP. Immobilized GST-PKI (64 μg) was incubated with CRM1 (76 μg) without (lanes 1–4) or with (lanes 5–8) RanGTP (78 μg). Bound and unbound proteins were analysed by SDS–PAGE and Coomassie staining. SDS–PAGE of input proteins shows that the same amount of CRM1 (either wild type or mutant) was used in each experiment. The mutation(s) designed to disrupt the hydrophobic interactions between HEAT9 loop and the inner surface of HEAT repeats 11 and 12 strengthened the binding of CRM1 to PKI (NES-cargo) in the absence of RanGTP. (C, D) The mutations of the hydrophobic residues of HEAT9 loop also substantially reduced the rate of RanBP1-accelerated NES release. Preincubated yeast CRM1-CFP:YFP-NES (PKI residues 38–47):RanGTP mixture (0.4 μM CRM1 (wild type (red) or V441D (green) or V441A/L442A (orange) or V443D (magenta))-CFP, 2.0 μM YFP-NES, 6.0 μM RanGTP) was rapidly mixed with buffer alone (blue), or with 8 μM RanBP1.
Figure 6
Functional significance of the interaction interface between CRM1 and RanBP1 in CRM1:RanBP1:RanGTP complex. (A) A close-up view of the interface between RanBP1 and CRM1. Yellow, CRM1 (only HEAT15 is shown for clarity); green, RanBP1; blue, the C-terminal region of Ran. Pro754CRM1 makes van der Waals contact with RanBP1. (B) A pulldown assay of the assembly of CRM1:NES:RanGTP complex. Immobilized 93 μg GST-RanGTP was incubated with 39 μg CRM1 (wild type or P754D mutant) and 20 μg CFP-YFP-NES (a Leu-rich NES of MVM NS2 protein; Askjaer et al, 1999). (C) A pulldown assay of the assembly of CRM1:RanBP1:RanGTP complex. Immobilized 93 μg GST-RanGTP was incubated with 39 μg CRM1 (wild type or P754D mutant) and 20 μg RanBP1. (D, E) NES dissociation kinetics. Preincubated CRM1(P754D)-CFP:YFP-NES (PKI residues 38–47):RanGTP mixture (0.4 μM CRM1(P754D)-CFP, 2.0 μM YFP-NES, 6.0 μM RanGTP) was rapidly mixed with buffer alone (blue) or with 80 μM PKI (green) or with 8 μM RanBP1 (red).
Figure 7
Primarily nuclear RanBDs of RanBP3 (Yrb2p) or Nup50 (Nup2p) do not displace NES-cargo from CRM1 and RanGTP. NES dissociation kinetics was measured by a FRET-based assay. (A) Preincubated human CRM1-CFP:YFP-NES (PKI residues 38–47):RanGTP mixture (0.4 μM CRM1-CFP, 2.0 μM YFP-NES, 6.0 μM RanGTP) was rapidly mixed with 80 μM PKI (green) or with 8 μM RanBD of human RanBP3b (residues 312–448) (orange), and then the time-dependent change in CFP-YFP FRET signal was recorded. (B) Preincubated yeast CRM1-CFP:YFP-NES (PKI residues 38–47):RanGTP mixture (0.4 μM CRM1-CFP, 2.0 μM YFP-NES, 6.0 μM RanGTP) was rapidly mixed with 80 μM PKI (green) or with 8 μM RanBD of yeast Yrb2p (residues 195–327) (orange). (C) Preincubated human CRM1-CFP:YFP-NES (PKI residues 38–47):RanGTP mixture (0.4 μM CRM1-CFP, 2.0 μM YFP-NES, 6.0 μM RanGTP) was rapidly mixed with 80 μM PKI (green) or with 8 μM RanBD of human Nup50 (residues 320–468) (orange). (D) Preincubated yeast CRM1-CFP:YFP-NES (PKI residues 38–47):RanGTP mixture (0.4 μM CRM1-CFP, 2.0 μM YFP-NES, 6.0 μM RanGTP) was rapidly mixed with 80 μM PKI (green) or with 8 μM RanBD of yeast Nup2p (residues 566–720) (orange).
Similar articles
- Atomic basis of CRM1-cargo recognition, release and inhibition.
Fung HY, Chook YM. Fung HY, et al. Semin Cancer Biol. 2014 Aug;27:52-61. doi: 10.1016/j.semcancer.2014.03.002. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631835 Free PMC article. Review. - A 2.1-Å-resolution crystal structure of unliganded CRM1 reveals the mechanism of autoinhibition.
Saito N, Matsuura Y. Saito N, et al. J Mol Biol. 2013 Jan 23;425(2):350-64. doi: 10.1016/j.jmb.2012.11.014. Epub 2012 Nov 16. J Mol Biol. 2013. PMID: 23164569 - Structural insights into how Yrb2p accelerates the assembly of the Xpo1p nuclear export complex.
Koyama M, Shirai N, Matsuura Y. Koyama M, et al. Cell Rep. 2014 Nov 6;9(3):983-95. doi: 10.1016/j.celrep.2014.09.052. Epub 2014 Oct 30. Cell Rep. 2014. PMID: 25437554 - Electrostatic interactions involving the extreme C terminus of nuclear export factor CRM1 modulate its affinity for cargo.
Fox AM, Ciziene D, McLaughlin SH, Stewart M. Fox AM, et al. J Biol Chem. 2011 Aug 19;286(33):29325-29335. doi: 10.1074/jbc.M111.245092. Epub 2011 Jun 27. J Biol Chem. 2011. PMID: 21708948 Free PMC article. - Allosteric control of the exportin CRM1 unraveled by crystal structure analysis.
Monecke T, Dickmanns A, Ficner R. Monecke T, et al. FEBS J. 2014 Sep;281(18):4179-94. doi: 10.1111/febs.12842. Epub 2014 Jun 6. FEBS J. 2014. PMID: 24823279 Free PMC article. Review.
Cited by
- Kinesin-1 and mitochondrial motility control by discrimination of structurally equivalent but distinct subdomains in Ran-GTP-binding domains of Ran-binding protein 2.
Patil H, Cho KI, Lee J, Yang Y, Orry A, Ferreira PA. Patil H, et al. Open Biol. 2013 Mar 27;3(3):120183. doi: 10.1098/rsob.120183. Open Biol. 2013. PMID: 23536549 Free PMC article. - Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia.
Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, Goettl V, Mahoney E, Berglund C, Gupta S, Farmer A, Mani R, Johnson AJ, Lucas D, Mo X, Daelemans D, Sandanayaka V, Shechter S, McCauley D, Shacham S, Kauffman M, Chook YM, Byrd JC. Lapalombella R, et al. Blood. 2012 Nov 29;120(23):4621-34. doi: 10.1182/blood-2012-05-429506. Epub 2012 Oct 3. Blood. 2012. PMID: 23034282 Free PMC article. - A cellular reporter to evaluate CRM1 nuclear export activity: functional analysis of the cancer-related mutant E571K.
García-Santisteban I, Arregi I, Alonso-Mariño M, Urbaneja MA, Garcia-Vallejo JJ, Bañuelos S, Rodríguez JA. García-Santisteban I, et al. Cell Mol Life Sci. 2016 Dec;73(24):4685-4699. doi: 10.1007/s00018-016-2292-0. Epub 2016 Jun 16. Cell Mol Life Sci. 2016. PMID: 27312238 Free PMC article. - BioCreative VI Precision Medicine Track system performance is constrained by entity recognition and variations in corpus characteristics.
Chen Q, Panyam NC, Elangovan A, Verspoor K. Chen Q, et al. Database (Oxford). 2018 Jan 1;2018:bay122. doi: 10.1093/database/bay122. Database (Oxford). 2018. PMID: 30576491 Free PMC article. - Atomic basis of CRM1-cargo recognition, release and inhibition.
Fung HY, Chook YM. Fung HY, et al. Semin Cancer Biol. 2014 Aug;27:52-61. doi: 10.1016/j.semcancer.2014.03.002. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631835 Free PMC article. Review.
References
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363–366 - PubMed
- Collaborative Computational Project Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Cryst D 50: 760–763 - PubMed
- Conti E, Müller CW, Stewart M (2006) Karyopherin flexibility in nucleocytoplasmic transport. Curr Opin Struct Biol 16: 237–244 - PubMed
- Cook AG, Fukuhara N, Jinek M, Conti E (2009) Structures of the tRNA export factor in the nuclear and cytosolic states. Nature 461: 60–65 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous