Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis - PubMed (original) (raw)
. 2010 Jul;177(1):415-23.
doi: 10.2353/ajpath.2010.090863. Epub 2010 May 20.
Lynette M Sholl, Michael Peyton, John Reilly, Christopher Ware, Lenora Davis, Natalie Vena, Dyane Bailey, Beow Y Yeap, Michelangelo Fiorentino, Azra H Ligon, Bo-Sheng Pan, Victoria Richon, John D Minna, Adi F Gazdar, Giulio Draetta, Silvano Bosari, Lucian R Chirieac, Bart Lutterbach, Massimo Loda
Affiliations
- PMID: 20489150
- PMCID: PMC2893683
- DOI: 10.2353/ajpath.2010.090863
Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis
Elisa Benedettini et al. Am J Pathol. 2010 Jul.
Abstract
Most non-small cell lung cancer (NSCLC) patients harboring activating epidermal growth factor receptor (EGFR) mutations respond to tyrosine kinase inhibitor (TKI) therapy. However, about 30% exhibit primary resistance to EGFR TKI therapy. Here we report that Met protein expression and phosphorylation were associated with primary resistance to EGFR TKI therapy in NSCLC patients harboring EGFR mutations, implicating Met as a de novo mechanism of resistance. In a separate patient cohort, Met expression and phosphorylation were also associated with development of NSCLC brain metastasis and were selectively enriched in brain metastases relative to paired primary lung tumors. A similar metastasis-specific activation of Met occurred in vitro in the isogenous cell lines H2073 and H1993, which are derived from the primary lung tumor and a metastasis, respectively, from the same patient. We conclude that Met activation is found in NSCLC before EGFR-targeted therapy and is associated with both primary resistance to EGFR inhibitor therapy and with the development of metastases. If confirmed in larger cohorts, our analysis suggests that patient tumors harboring both Met activation and EGFR mutation could potentially benefit from early intervention with a combination of EGFR and Met inhibitors.
Figures
Figure 1
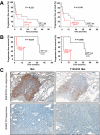
Met expression and phosphorylation in EGFR mutation containing NSCLC tumors are associated with poor response to EGFR TKI therapy. Kaplan–Meier curves of progression-free survival (PFS) for Met and Y1234/35 Met in 23 NSCLC patients before EGFR inhibitor therapy (A), and the subset of patients (n = 10) harboring EGFR mutations (B). C: Top panels (EGFR TKI nonresponsive) indicate elevated Met and Y1234/35 Met in the tumor specimen of a nonresponsive patient (Patient eight, Table one, PFS of three months); bottom panels (EGFR TKI responsive) indicate undetectable Met and Y1234/35 Met in the tumor from a responsive patient tumor (PFS = 40 months, patient five, Table one). FISH analysis revealed low Met copy gain in both patients (Table 1).
Figure 2
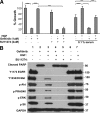
A: Gefitinib-induced growth inhibition of HCC827 is rescued by HGF. Gefitinib (1 μmol/L) addition was followed by addition of 50 ng/ml HGF. SU11274 (2 μmol/L) addition blocked HGF-mediated rescue. HGF rescue was maintained in 0.1% serum as indicated. Cell growth was measured after four days of treatment and is shown relative to untreated cells. Data represent mean ± SEM. ***P < 0.001. B: HGF restores signal transduction pathways in Gefitinib-treated cells. Gefitinib (1 μmol/L) was added to HCC827 cells, followed by addition of 50 ng/ml HGF. Seventy-two hours later, lysates were prepared and analyzed by SDS-PAGE and Western blotted for the indicated phospho proteins.
Figure 3
Colocalization of Met phosphorylation and EGFR mutation. A and B: Exon 19 EGFR deletion, detected with a delEGFR-specific antibody. C and D: Met Y1234/35 phosphorylation. IHC images on consecutive sections (patient 2, Table 1) reveal colocalization of delEGFR and Y1234/35 Met. Arrowheads indicate regions of positivity for both antibodies; arrows indicate regions harboring EGFR deletion but not Met activation. B and D are magnification (×400) of A and C (×100), respectively.
Figure 4
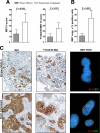
Met expression, phosphorylation, and gene copy gain are enriched in brain metastases relative to their paired NSCLC. A: Met and Y1234/35 Met were assessed by immunohistochemistry (H score) in primary NSCLCs and paired brain metastases. Both Met expression (P = 0.003) and Y1234/35 Met phosphorylation (P = 0.031) are significantly upregulated in metastases. Data represent mean ± SEM. B: Prevalence of MET copy gain (factoring MET copy number gain and % positive cells as in Materials and Methods) is higher in the metastatic lesions relative to the paired primary NSCLCs (P = 0.024). C: Met IHC (magnification ×200) and FISH analysis on a primary NSCLC and its paired brain metastasis (patient 10, Table 1). Met and Y1234/35 Met staining was heterogeneous/focal in the primary cancer but more widespread in the paired brain metastasis. MET FISH reveals low copy gain (MET copies, n = 3 to 5) in the primary lung tumor, and high copy gain (MET copies, n = 6 to 10) in the metastatic lesion.
Figure 5
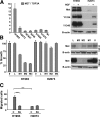
MET is amplified and constitutively activated in the metastasis-derived H1993 cell line but not in the paired primary lung tumor–derived H2073 cell line. A: Quantitative PCR for MET copy number. MET is not amplified in H2073 cells but is amplified in H1993 cells. Data represent mean ± SEM. Immunoblot reveals elevated Met expression and Y1234/35 and Y1349 phosphorylation in H1993 cells but not in H2073 cells. B-actin is the loading control. B: Growth of H2073 cells is not inhibited by Met shRNA, whereas H1993 cell growth is inhibited. Data represent mean ± SEM. Immunoblot revealing efficient Met knockdown in H2073 and H1993 cells treated with Met shRNA M3 and less efficient knockdown with Met shRNA M2 and M1. L indicates Luciferase shRNA; 0, no shRNA. C: Migration assay was performed in the indicated cell lines as described in Methods. 0, initial time point; 72, 72 hours later; SU, addition of 2.5 μmol/L SU11274 Met inhibitor. Data represent mean ± SEM. *P < 0.5, ***P < 0.001. Results are representative of three independent experiments.
Similar articles
- Clonal MET Amplification as a Determinant of Tyrosine Kinase Inhibitor Resistance in Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer.
Lai GGY, Lim TH, Lim J, Liew PJR, Kwang XL, Nahar R, Aung ZW, Takano A, Lee YY, Lau DPX, Tan GS, Tan SH, Tan WL, Ang MK, Toh CK, Tan BS, Devanand A, Too CW, Gogna A, Ong BH, Koh TPT, Kanesvaran R, Ng QS, Jain A, Rajasekaran T, Yuan J, Lim TKH, Lim AST, Hillmer AM, Lim WT, Iyer NG, Tam WL, Zhai W, Tan EH, Tan DSW. Lai GGY, et al. J Clin Oncol. 2019 Apr 10;37(11):876-884. doi: 10.1200/JCO.18.00177. Epub 2019 Jan 24. J Clin Oncol. 2019. PMID: 30676858 - Crizotinib with or without an EGFR-TKI in treating EGFR-mutant NSCLC patients with acquired MET amplification after failure of EGFR-TKI therapy: a multicenter retrospective study.
Wang W, Wang H, Lu P, Yu Z, Xu C, Zhuang W, Song Z. Wang W, et al. J Transl Med. 2019 Feb 21;17(1):52. doi: 10.1186/s12967-019-1803-9. J Transl Med. 2019. PMID: 30791921 Free PMC article. - Leukocyte Cell-Derived Chemotaxin 2 Retards Non-Small Cell Lung Cancer Progression Through Antagonizing MET and EGFR Activities.
Hung WY, Chang JH, Cheng Y, Chen CK, Chen JQ, Hua KT, Cheng CW, Hsiao M, Chung CL, Lee WJ, Chien MH. Hung WY, et al. Cell Physiol Biochem. 2018;51(1):337-355. doi: 10.1159/000495233. Epub 2018 Nov 19. Cell Physiol Biochem. 2018. PMID: 30453282 - Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway.
Nguyen KS, Kobayashi S, Costa DB. Nguyen KS, et al. Clin Lung Cancer. 2009 Jul;10(4):281-9. doi: 10.3816/CLC.2009.n.039. Clin Lung Cancer. 2009. PMID: 19632948 Free PMC article. Review. - Capmatinib for the treatment of non-small cell lung cancer.
Vansteenkiste JF, Van De Kerkhove C, Wauters E, Van Mol P. Vansteenkiste JF, et al. Expert Rev Anticancer Ther. 2019 Aug;19(8):659-671. doi: 10.1080/14737140.2019.1643239. Epub 2019 Aug 1. Expert Rev Anticancer Ther. 2019. PMID: 31368815 Review.
Cited by
- Modeling lung adenocarcinoma metastases using patient-derived organoids.
Liu Y, Lankadasari M, Rosiene J, Johnson KE, Zhou J, Bapat S, Chow-Tsang LL, Tian H, Mastrogiacomo B, He D, Connolly JG, Lengel HB, Caso R, Dunne EG, Fick CN, Rocco G, Sihag S, Isbell JM, Bott MJ, Li BT, Lito P, Brennan CW, Bilsky MH, Rekhtman N, Adusumilli PS, Mayo MW, Imielinski M, Jones DR. Liu Y, et al. Cell Rep Med. 2024 Oct 15;5(10):101777. doi: 10.1016/j.xcrm.2024.101777. Cell Rep Med. 2024. PMID: 39413736 Free PMC article. - Unveiling the Role of HGF/c-Met Signaling in Non-Small Cell Lung Cancer Tumor Microenvironment.
Yao S, Liu X, Feng Y, Li Y, Xiao X, Han Y, Xia S. Yao S, et al. Int J Mol Sci. 2024 Aug 22;25(16):9101. doi: 10.3390/ijms25169101. Int J Mol Sci. 2024. PMID: 39201787 Free PMC article. Review. - Oncogenic STAT Transcription Factors as Targets for Cancer Therapy: Innovative Strategies and Clinical Translation.
Wang W, Lopez McDonald MC, Hariprasad R, Hamilton T, Frank DA. Wang W, et al. Cancers (Basel). 2024 Mar 31;16(7):1387. doi: 10.3390/cancers16071387. Cancers (Basel). 2024. PMID: 38611065 Free PMC article. Review. - NOTCH1 and CREBBP co-mutations negatively affect the benefit of adjuvant therapy in completely resected EGFR-mutated NSCLC: translational research of phase III IMPACT study.
Ikeda S, Tsuboi M, Sakai K, Misumi T, Akamatsu H, Shoda H, Sakakura N, Nakamura A, Ohde Y, Hayashi H, Okishio K, Okada M, Yoshino I, Okami J, Takahashi K, Ikeda N, Tanahashi M, Tambo Y, Saito H, Toyooka S, Inokawa H, Chen-Yoshikawa T, Yokoyama T, Okamoto T, Yanagitani N, Oki M, Takahama M, Sawa K, Tada H, Nakagawa K, Mitsudomi T, Nishio K. Ikeda S, et al. Mol Oncol. 2024 Feb;18(2):305-316. doi: 10.1002/1878-0261.13542. Epub 2023 Oct 28. Mol Oncol. 2024. PMID: 37864465 Free PMC article. - Targeting MET Amplification: Opportunities and Obstacles in Therapeutic Approaches.
Kumaki Y, Oda G, Ikeda S. Kumaki Y, et al. Cancers (Basel). 2023 Sep 14;15(18):4552. doi: 10.3390/cancers15184552. Cancers (Basel). 2023. PMID: 37760522 Free PMC article. Review.
References
- Asahina H, Yamazaki K, Kinoshita I, Sukoh N, Harada M, Yokouchi H, Ishida T, Ogura S, Kojima T, Okamoto Y, Fujita Y, Dosaka-Akita H, Isobe H, Nishimura M. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer. 2006;95:998–1004. - PMC - PubMed
- Chang AY. The role of gefitinib in the management of Asian patients with non-small cell lung cancer. Expert Opin Investig Drugs. 2008;17:401–411. - PubMed
- Inoue A, Suzuki T, Fukuhara T, Maemondo M, Kimura Y, Morikawa N, Watanabe H, Saijo Y, Nukiwa T. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol. 2006;24:3340–3346. - PubMed
- Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J, Skarin AT, Meyerson M, Holmes AJ, Borras AM, Freidlin B, Ostler PA, Lucca J, Lynch TJ, Johnson BE, Janne PA. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–766. - PubMed
- Sunaga N, Tomizawa Y, Yanagitani N, Iijima H, Kaira K, Shimizu K, Tanaka S, Suga T, Hisada T, Ishizuka T, Saito R, Dobashi K, Mori M. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer. 2007;56:383–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous