Failure of regulation results in an amplified oxidation burst by neutrophils in children with primary nephrotic syndrome - PubMed (original) (raw)
Comparative Study
Failure of regulation results in an amplified oxidation burst by neutrophils in children with primary nephrotic syndrome
R Bertelli et al. Clin Exp Immunol. 2010.
Abstract
The mechanism responsible for proteinuria in non-genetic idiopathic nephrotic syndrome (iNS) is unknown. Animal models suggest an effect of free radicals on podocytes, and indirect evidence in humans confirm this implication. We determined the oxidative burst by blood CD15+ polymorphonucleates (PMN) utilizing the 5-(and-6)-carboxy-2',7'-dichlorofluorescin diacetate (DCF-DA) fluorescence assay in 38 children with iNS. Results were compared with PMN from normal subjects and patients with renal pathologies considered traditionally to be models of oxidative stress [six anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis, seven post-infectious glomerulonephritis]. Radicals of oxygen (ROS) production was finally determined in a patient with immunodeficiency, polyendocrinopathy, enteropathy X-linked (IPEX) and in seven iNS children after treatment with Rituximab. Results demonstrated a 10-fold increase of ROS production by resting PMN in iNS compared to normal PMN. When PMN were separated from other cells, ROS increased significantly in all conditions while a near-normal production was restored by adding autologous cells and/or supernatants in controls, vasculitis and post-infectious glomerulonephritis but not in iNS. Results indicated that the oxidative burst was regulated by soluble factors and that this regulatory circuit was altered in iNS. PMN obtained from a child with IPEX produced 100 times more ROS during exacerbation of clinical symptoms and restored to a near normal-level in remission. Rituximab decreased ROS production by 60%. In conclusion, our study shows that oxidant production is increased in iNS for an imbalance between PMN and other blood cells. Regulatory T cells (Tregs) and CD20 are probably involved in this regulation. Overall, our observations reinforce the concept that oxidants deriving from PMN are implicated in iNS.
Figures
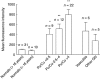
Fig. 1
Radicals of oxygen (ROS) production by resting polymorphonucleates (PMN). 5-(and-6)-carboxy-2′,7′-dichlorofluorescin diacetate (DCF-DA) fluorescence assay was performed with whole blood in which PMN (CD15+ cells) were analysed as separated gates by fluorescence activated cell sorter (FACS). Thirty-one normal subjects and 38 children with idiopathic nephrotic syndrome (iNS) were considered in this first set of experiments. Patients were evaluated in different clinical phases as defined by their urinary protein/urinary creatinine ratio (Pu/Cu) (remission Pu/Cu < 0·5; relapse Pu/Cu > 4; intermediate Pu/Cu 0·5–4). Six patients with renal vasculitis and five with other glomerulonephritis were evaluated in the same experimental conditions. Statistical analysis performed with parametric tests (one-way analysis of variance) showed significant difference in ROS production *P < 0·05; **P < 0·01.
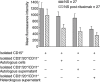
Fig. 2
Radicals of oxygen (ROS) production by polymorphonucleates (PMN) isolated from other cells. (a) 5-(and-6)-carboxy-2′,7′-dichlorofluorescin diacetate (DCF-DA) fluorescence assay performed utilizing whole blood as in Fig. 1. In other experiments, PMN (CD15+ cells) were first isolated by dextran sedimentation in various experimental conditions: comparison of PMN obtained from normal donors and from children with active idiopathic nephrotic syndrome (iNS); (b) comparison of isolated PMN obtained from normal subjects and from patients with iNS. DCF-DA analysis was performed with isolated PMN alone and/or in presence of autologous–heterologous cells and supernatants deriving from CD3+, CD11+ and CD20+ cells; (c) patients with vasculitis and post-streptococcal glomerulonephritis. Statistical analysis performed with parametric tests (one-way analysis of variance) showed significant difference in ROS production *P < 0·01.
Fig. 3
Radicals of oxygen (ROS) production by polymorphonucleates (PMN) in an immunodeficiency, polyendocrinopathy, enteropathy X-linked (IPEX) child. 5-(and-6)-carboxy-2′,7′-dichlorofluorescin diacetate (DCF-DA) fluorescence assay performed with whole blood containing non-isolated PMN (CD15+ cells) in a child with immunodeficiency, polyendocrinopathy, enteropathy X-linked (IPEX) and his normal mother, carrier of the same mutation (p.Leu242Pro) of forkhead box P3 (FoxP3).
Fig. 4
Radicals of oxygen (ROS) production after Rituximab. 5-(and-6)-carboxy-2′,7′-dichlorofluorescin diacetate (DCF-DA) fluorescence assay performed with isolated polymorphonucleates (PMN) (CD15+ cells) in patients with idiopathic nephrotic syndrome (iNS) after an in vivo treatment with anti-CD20 antibodies (Rituximab). Isolated PMN were studied alone and in the presence of autologous–heterologous cells and supernatants deriving from CD3+, CD11+ and CD20+ cells.
Similar articles
- Regulation of innate immunity by the nucleotide pathway in children with idiopathic nephrotic syndrome.
Bertelli R, Bodria M, Nobile M, Alloisio S, Barbieri R, Montobbio G, Patrone P, Ghiggeri GM. Bertelli R, et al. Clin Exp Immunol. 2011 Oct;166(1):55-63. doi: 10.1111/j.1365-2249.2011.04441.x. Epub 2011 Jul 15. Clin Exp Immunol. 2011. PMID: 21762125 Free PMC article. - Toll-like receptor 3 expression and function in childhood idiopathic nephrotic syndrome.
Jamin A, Dehoux L, Dossier C, Fila M, Heming N, Monteiro RC, Deschênes G. Jamin A, et al. Clin Exp Immunol. 2015 Dec;182(3):332-45. doi: 10.1111/cei.12659. Epub 2015 Sep 30. Clin Exp Immunol. 2015. PMID: 26123900 Free PMC article. Clinical Trial. - Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: study protocol.
Ravani P, Bonanni A, Ghiggeri GM. Ravani P, et al. BMJ Open. 2017 Mar 17;7(3):e013319. doi: 10.1136/bmjopen-2016-013319. BMJ Open. 2017. PMID: 28314744 Free PMC article. Clinical Trial. - Rituximab in idiopathic nephrotic syndrome: does it make sense?
Cara-Fuentes G, Kairalla JA, Ishimoto T, Rivard C, Johnson RJ, Garin EH. Cara-Fuentes G, et al. Pediatr Nephrol. 2014 Aug;29(8):1313-9. doi: 10.1007/s00467-013-2534-4. Epub 2013 Jun 23. Pediatr Nephrol. 2014. PMID: 23793923 Free PMC article. Review. - Anti-CD20 Antibodies for Idiopathic Nephrotic Syndrome in Children.
Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM. Ravani P, et al. Clin J Am Soc Nephrol. 2016 Apr 7;11(4):710-20. doi: 10.2215/CJN.08500815. Epub 2015 Nov 19. Clin J Am Soc Nephrol. 2016. PMID: 26585985 Free PMC article. Review.
Cited by
- Effect of octreotide on oxidative stress in the erythrocyte and kidney tissue in adriamycin-induced experimental nephrotic syndrome model.
Cavdar S, Acar AG, Camyar A, Hür E, Sozmen EY, Sen S, Ozısık M, Akcay YD, Duman E, Gönen S, Akcicek F, Duman S. Cavdar S, et al. J Bras Nefrol. 2024 Jan-Mar;46(1):18-28. doi: 10.1590/2175-8239-JBN-2022-0180en. J Bras Nefrol. 2024. PMID: 37527531 Free PMC article. - Genetics of Childhood Steroid Sensitive Nephrotic Syndrome: An Update.
Lane BM, Cason R, Esezobor CI, Gbadegesin RA. Lane BM, et al. Front Pediatr. 2019 Jan 29;7:8. doi: 10.3389/fped.2019.00008. eCollection 2019. Front Pediatr. 2019. PMID: 30761277 Free PMC article. Review. - Molecular and Cellular Mechanisms for Proteinuria in Minimal Change Disease.
Bertelli R, Bonanni A, Caridi G, Canepa A, Ghiggeri GM. Bertelli R, et al. Front Med (Lausanne). 2018 Jun 11;5:170. doi: 10.3389/fmed.2018.00170. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29942802 Free PMC article. Review. - Genetics of childhood steroid-sensitive nephrotic syndrome.
Karp AM, Gbadegesin RA. Karp AM, et al. Pediatr Nephrol. 2017 Sep;32(9):1481-1488. doi: 10.1007/s00467-016-3456-8. Epub 2016 Jul 29. Pediatr Nephrol. 2017. PMID: 27470160 Free PMC article. Review. - Regulatory T cells and minimal change nephropathy: in the midst of a complex network.
Bertelli R, Bonanni A, Di Donato A, Cioni M, Ravani P, Ghiggeri GM. Bertelli R, et al. Clin Exp Immunol. 2016 Feb;183(2):166-74. doi: 10.1111/cei.12675. Epub 2015 Oct 12. Clin Exp Immunol. 2016. PMID: 26147676 Free PMC article. Review.
References
- Vincenti F, Ghiggeri GM. New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant. 2005;5:1179–85. - PubMed
- Lagrue G, Xheneumont S, Branellec A, Weil B. Letter: lymphokines and nephrotic syndrome. Lancet. 1975;1:271–2. - PubMed
- Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–60. - PubMed
- Ghiggeri GM, Artero M, Carraro M, Perfumo F. Permeability plasma factors in nephrotic syndrome: more than one factor, more than one inhibitor. Nephrol Dial Transplant. 2001;16:882–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources