Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A - PubMed (original) (raw)
Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A
Gregory F Sonnenberg et al. J Exp Med. 2010.
Abstract
IL-22 has both proinflammatory and tissue-protective properties depending on the context in which it is expressed. However, the factors that influence the functional outcomes of IL-22 expression remain poorly defined. We demonstrate that after administration of a high dose of bleomycin that induces acute tissue damage and airway inflammation and is lethal to wild-type (WT) mice, Th17 cell-derived IL-22 and IL-17A are expressed in the lung. Bleomycin-induced disease was ameliorated in Il22-/- mice or after anti-IL-22 monoclonal antibody (mAb) treatment of WT mice, indicating a proinflammatory/pathological role for IL-22 in airway inflammation. However, despite increased bleomycin-induced IL-22 production, Il17a-/- mice were protected from airway inflammation, suggesting that IL-17A may regulate the expression and/or proinflammatory properties of IL-22. Consistent with this, IL-17A inhibited IL-22 production by Th17 cells, and exogenous administration of IL-22 could only promote airway inflammation in vivo by acting in synergy with IL-17A. Anti-IL-22 mAb was delivered to Il17a-/- mice and was found to exacerbate bleomycin-induced airway inflammation, indicating that IL-22 is tissue protective in the absence of IL-17A. Finally, in an in vitro culture system, IL-22 administration protected airway epithelial cells from bleomycin-induced apoptosis, and this protection was reversed after coadministration of IL-17A. These data identify that IL-17A can regulate the expression, proinflammatory properties, and tissue-protective functions of IL-22, and indicate that the presence or absence of IL-17A governs the proinflammatory versus tissue-protective properties of IL-22 in a model of airway damage and inflammation.
Figures
Figure 1.
A Th17 response develops after bleomycin-induced airway inflammation. C57BL/6 mice were intratracheally instilled with PBS or bleomycin (Bleo) and sacrificed on day 8. (A) cDNA prepared from lungs was analyzed by RT-PCR for Il17a and Il22 expression. (B) Live lung cells from bleomycin-instilled mice were analyzed for the frequency of IL-17A+ and IL-22+ T cells. Populations are sequentially gated on total live cells (left), total cytokine-positive cells (middle), and TCRβ+ cells (right). (C) Total numbers of IL-17A+ and IL-22+ CD4+ T cells obtained from the lungs of PBS or bleomycin-treated mice. (D) Lung cell suspensions were stimulated with anti-CD3 mAb for 48 h, and supernatants were analyzed for IL-17A and IL-22 secretion by ELISA. (E) CD4+ T cells from the BAL and lung cells were analyzed for the frequency of IL-17A+ and IL-22+ cells by flow cytometry. (F) Frequency from individual mice of IL-17A+, IL-22+, and IL-17A+/IL-22+ CD4+ T cells in the BAL. All data are representative of three or more independent experiments with a minimum of three to four mice per group. Data shown are means ± SEM. Significance was determined using the Mann-Whitney U test. *, P < 0.05.
Figure 2.
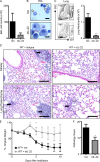
Administration of anti–IL-22 mAb protects mice from bleomycin-induced airway inflammation. C57BL/6 mice were intratracheally instilled with bleomycin, treated with an isotype control (Iso) or anti–IL-22 (αIL-22) mAb, and sacrificed on day 10. (A) BAL cell counts. (B and C) Neutrophil infiltration was assessed by H&E staining of BAL cell cytocentrifuge preparations (B; neutrophils are highlighted by white arrows) and by flow cytometry of lung cells for the frequency (left) and total number (right) of Gr-1+ cells (C). Bars, 10 µm. (D) H&E staining of histological lung sections demonstrating peribronchial leukocyte infiltrate (black arrows) and disruption of lung architecture (white arrows). Bars, 100 µm. (E) Weight loss of individual mice was plotted as a percentage of starting weight. (F) Total pathology score. All data are representative of three or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. *, P < 0.05; **, P < 0.01.
Figure 3.
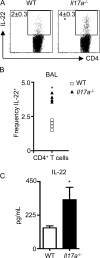
IL-17A partially regulates the expression of IL-22. WT and Il17a−/− mice were intratracheally instilled with bleomycin and sacrificed on day 10. (A) CD4+ BAL T cells were analyzed for the frequency of IL-22+ cells by flow cytometry. (B) Frequency of individual mice for IL-22+ CD4+ T cell increases in the BAL. (C) Lung cell suspensions were stimulated with anti-CD3 mAb for 48 h, and supernatants were analyzed for IL-22 secretion by ELISA. All data are representative of two or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. Significance was determined using the Mann-Whitney U test. *, P < 0.05.
Figure 4.
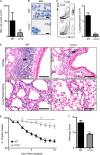
Il17a−/− mice are protected from bleomycin-induced pulmonary inflammation. WT or Il17a−/− mice were intratracheally instilled with bleomycin and sacrificed on day 10. (A) BAL cell counts. (B and C) Neutrophil infiltration was assessed by H&E staining of BAL cell cytocentrifuge preparations (B; neutrophils are highlighted with white arrows) and by flow cytometry of lung cells for the frequency (left) and total number (right) of Gr-1+ cells (C). Bars, 10 µm. (D) H&E staining of histological lung sections demonstrating peribronchial leukocyte infiltrate (black arrows) and disruption in lung architecture (white arrows). Bars, 100 µm. (E) Weight loss of individual mice was plotted as a percentage of starting weight. (F) Total pathology score. All data are representative of three or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. **, P < 0.01; ***, P < 0.001.
Figure 5.
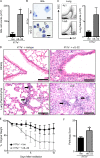
Blockade of IL-22 reverses protection of Il17a−/− mice from bleomycin-induced pulmonary inflammation. Il17a−/− mice were intratracheally instilled with bleomycin, treated with an isotype control (Iso) or anti–IL-22 (αIL-22) mAb, and sacrificed on day 10. (A) BAL cell counts. (B and C) Neutrophil infiltration was assessed by H&E staining of BAL cell cytocentrifuge preparations (B; highlighted by white arrows) and by flow cytometry of lung cells for the frequency (left) and total number (right) of Gr-1+ cells (C). Bars, 10 µm. (D) H&E staining of histological lung sections demonstrating peribronchial leukocyte infiltrate (black arrows), disruption in lung architecture (white arrows), and epithelial hyperplasia (gray arrow). Bars, 100 µm. (E) Weight loss of individual mice was plotted as a percentage of starting weight. (F) Total pathology score. All data are representative of three or more independent experiments with a minimum of three to five mice per group. Data shown are means ± SEM. *, P < 0.05.
Figure 6.
IL-17A regulates the tissue-protective properties of IL-22. (A) cDNA prepared from MLE cells and splenocytes (Spl) was examined for the presence of Il22ra transcripts by RT-PCR. (B) Immunoblot of MLE cell and total splenocyte lysates with antibody for IL-22Rα and β-actin. (C) MLE cells were stimulated with 10 ng/ml rIL-22 for the indicated times before lysis and immunostaining for phopho-STAT3 (pSTAT3) and total STAT3. (D) For imaging, MLE cell were treated overnight with bleomycin in the absence (Bleo) or presence (+rIL-22) of rIL-22. (E and F) Induction of apoptosis was identified by in situ TUNEL staining (E) or flow cytometry analysis for the frequency of annexin V+ cells (F). Bars, 10 µm. (G) Frequency of annexin V+ cells with the addition of increasing concentrations of rIL-22. (H) cDNA from bleomycin-treated MLE cells in the presence or absence of 100 ng/ml rIL-22 was prepared and analyzed for Bcl2 and Bcl2l1 transcripts by RT-PCR. (I) Percent protection with the addition of 10 ng/ml rIL-17A and 10 ng/ml rIL-22 was determined based on the frequency of annexin V+ cells above or below that obtained with bleomycin alone (set at 0). (J) In situ TUNEL staining of paraffin-embedded lung tissue. Bars, 10 µm. Data from in vitro studies are representative of two or more independent experiments with triplicate wells per condition. Data shown are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Similar articles
- Role of neutralizing anti-murine interleukin-17A monoclonal antibody on chronic ozone-induced airway inflammation in mice.
Zhang M, Fei X, Zhang GQ, Zhang PY, Li F, Bao WP, Zhang YY, Zhou X. Zhang M, et al. Biomed Pharmacother. 2016 Oct;83:247-256. doi: 10.1016/j.biopha.2016.06.041. Epub 2016 Jul 2. Biomed Pharmacother. 2016. PMID: 27380433 - Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice.
Chen Y, Li C, Weng D, Song L, Tang W, Dai W, Yu Y, Liu F, Zhao M, Lu C, Chen J. Chen Y, et al. Toxicol Appl Pharmacol. 2014 Feb 15;275(1):62-72. doi: 10.1016/j.taap.2013.11.012. Epub 2013 Nov 26. Toxicol Appl Pharmacol. 2014. PMID: 24291675 - Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis.
Lei L, Zhao C, Qin F, He ZY, Wang X, Zhong XN. Lei L, et al. Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):14-22. Epub 2016 Jan 11. Clin Exp Rheumatol. 2016. PMID: 26750756 - The roles of IL-17A in inflammatory immune responses and host defense against pathogens.
Iwakura Y, Nakae S, Saijo S, Ishigame H. Iwakura Y, et al. Immunol Rev. 2008 Dec;226:57-79. doi: 10.1111/j.1600-065X.2008.00699.x. Immunol Rev. 2008. PMID: 19161416 Review. - Interleukin-17A: Potential mediator and therapeutic target in hypertension.
Rodrigues-Diez RR, Tejera-Muñoz A, Orejudo M, Marquez-Exposito L, Santos-Sanchez L, Rayego-Mateos S, Cantero-Navarro E, Tejedor-Santamaria L, Marchant V, Ortiz A, Egido J, Mezzano S, Selgas R, Navarro-González JF, Valdivielso JM, Lavoz C, Ruiz-Ortega M. Rodrigues-Diez RR, et al. Nefrologia (Engl Ed). 2021 May-Jun;41(3):244-257. doi: 10.1016/j.nefroe.2021.06.003. Epub 2021 Jul 14. Nefrologia (Engl Ed). 2021. PMID: 36166242 Review.
Cited by
- Role of HIF-1α-Activated IL-22/IL-22R1/Bmi1 Signaling Modulates the Self-Renewal of Cardiac Stem Cells in Acute Myocardial Ischemia.
Lee W, Lin SL, Chiang CS, Chen JY, Chieng WW, Huang SR, Chang TY, Linju Yen B, Hung MC, Chang KC, Lee HT, Jeng LB, Shyu WC. Lee W, et al. Stem Cell Rev Rep. 2024 Nov;20(8):2194-2214. doi: 10.1007/s12015-024-10774-8. Epub 2024 Sep 12. Stem Cell Rev Rep. 2024. PMID: 39264501 Free PMC article. - A targetable type III immune response with increase of IL-17A expressing CD4+ T cells is associated with immunotherapy-induced toxicity in melanoma.
Dimitriou F, Cheng PF, Saltari A, Schaper-Gerhardt K, Staeger R, Haunerdinger V, Sella F, Tastanova A, Urban C, Dettwiler S, Mihic-Probst D, Matter CM, Michielin O, Gutzmer R, Long GV, Becher B, Levesque MP, Dummer R. Dimitriou F, et al. Nat Cancer. 2024 Sep;5(9):1390-1408. doi: 10.1038/s43018-024-00810-4. Epub 2024 Aug 29. Nat Cancer. 2024. PMID: 39210005 Free PMC article. - Role of the type 3 cytokines IL-17 and IL-22 in modulating metabolic dysfunction-associated steatotic liver disease.
Abdelnabi MN, Hassan GS, Shoukry NH. Abdelnabi MN, et al. Front Immunol. 2024 Aug 2;15:1437046. doi: 10.3389/fimmu.2024.1437046. eCollection 2024. Front Immunol. 2024. PMID: 39156888 Free PMC article. Review. - Endogenous IL-22 contributes to the pathogenesis of salivary gland dysfunction in the non-obese diabetic model of Sjögren's syndrome.
Felix FA, Zhou J, Li D, Onodera S, Yu Q. Felix FA, et al. Mol Immunol. 2024 Sep;173:20-29. doi: 10.1016/j.molimm.2024.06.010. Epub 2024 Jul 16. Mol Immunol. 2024. PMID: 39018744 - Ferroptosis-Regulated Natural Products and miRNAs and Their Potential Targeting to Ferroptosis and Exosome Biogenesis.
Chuang YT, Yen CY, Chien TM, Chang FR, Tsai YH, Wu KC, Tang JY, Chang HW. Chuang YT, et al. Int J Mol Sci. 2024 May 31;25(11):6083. doi: 10.3390/ijms25116083. Int J Mol Sci. 2024. PMID: 38892270 Free PMC article. Review.
References
- Alonzi T., Maritano D., Gorgoni B., Rizzuto G., Libert C., Poli V. 2001. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol. Cell. Biol. 21:1621–1632 10.1128/MCB.21.5.1621-1632.2001 - DOI - PMC - PubMed
- Braun R.K., Ferrick C., Neubauer P., Sjoding M., Sterner-Kock A., Kock M., Putney L., Ferrick D.A., Hyde D.M., Love R.B. 2008. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 31:167–179 10.1007/s10753-008-9062-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- T32AI007532-08/AI/NIAID NIH HHS/United States
- AI083480/AI/NIAID NIH HHS/United States
- AI74878/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- AI61570/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- T32 AI007532/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases