Structural and functional analysis of the interaction between the nucleoporin Nup98 and the mRNA export factor Rae1 - PubMed (original) (raw)
Structural and functional analysis of the interaction between the nucleoporin Nup98 and the mRNA export factor Rae1
Yi Ren et al. Proc Natl Acad Sci U S A. 2010.
Abstract
The export of mRNAs is a multistep process, involving the packaging of mRNAs into messenger ribonucleoprotein particles (mRNPs), their transport through nuclear pore complexes, and mRNP remodeling events prior to translation. Ribonucleic acid export 1 (Rae1) and Nup98 are evolutionarily conserved mRNA export factors that are targeted by the vesicular stomatitis virus matrix protein to inhibit host cell nuclear export. Here, we present the crystal structure of human Rae1 in complex with the Gle2-binding sequence (GLEBS) of Nup98 at 1.65 A resolution. Rae1 forms a seven-bladed beta-propeller with several extensive surface loops. The Nup98 GLEBS motif forms an approximately 50-A-long hairpin that binds with its C-terminal arm to an essentially invariant hydrophobic surface that extends over the entire top face of the Rae1 beta-propeller. The C-terminal arm of the GLEBS hairpin is necessary and sufficient for Rae1 binding, and we identify a tandem glutamate element in this arm as critical for complex formation. The Rae1*Nup98(GLEBS) surface features an additional conserved patch with a positive electrostatic potential, and we demonstrate that the complex possesses single-stranded RNA-binding capability. Together, these data suggest that the Rae1*Nup98 complex directly binds to the mRNP at several stages of the mRNA export pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Structural overview of the Rae1•Nup98GLEBS complex. (A) Domain organization of human Rae1 and human Nup98. For Rae1, the NTE (red) and the seven WD40 repeats (orange) are indicated. For Nup98, the GLEBS motif (magenta), the FG-repeat region (gray), the unstructured region (dark gray), the auto-proteolytic domain (pink), and the C-terminal 6 kDa fragment (light gray) that is removed by cotranslational proteolysis are indicated. In an alternatively spliced version, the Nup98-96 precursor, the 6 kDa fragment is replaced by Nup96, a protein that is embedded in the symmetric NPC core (
Fig. S1
). The arrows indicate the sites of autoproteolytic cleavage. (B) Ribbon representation of the Rae1•Nup98GLEBS complex. The Nup98 GLEBS motif is indicated in magenta. For Rae1, the β-propeller domain (blue), the NTE (red), the 5D6A loop (green), and the 7BC loop (yellow) are indicated. A 90° rotated view is shown on the right. (C) Schematic representation of the Rae1•Nup98GLEBS structure, colored according to B. The blades of the Rae1 β-propeller are labeled from one to seven. An asterisk indicates the Velcro-closure β-strand (17).
Fig. 2.
Surface properties of Rae1 and the Rae1•Nup98GLEBS complex. (A) Surface renditions of Rae1 and Rae1•Nup98GLEBS. The Rae1 surface that mediates the association with the Nup98 GLEBS motif is colored in magenta; the remainder is colored in blue. In the Rae1•Nup98GLEBS complex, the Rae1 and the Nup98 GLEBS motif surfaces are colored in blue and light magenta, respectively (Right). As a reference, a ribbon representation of the Rae1•Nup98GLEBS complex is shown and corresponds to the orientation of the left panel. The black line in the left panel indicates the location of the side view surface. (B) The surface representations of Rae1 and the Rae1•Nup98GLEBS complex are colored according to multispecies sequence alignments (Fig. 4_A_ and
Fig. S3
). The conservation at each position is mapped onto the surface and shaded in a color gradient from yellow (60% similarity) to red (100% identity). (C) Rae1 and Rae1•Nup98GLEBS surface renditions, colored according to the electrostatic potential, ranging from red (-10 kB T/e) to blue (+10 kB T/e).
Fig. 4.
The tandem glutamate element of the Nup98 GLEBS motif is essential for the interaction with Rae1. (A) Multispecies sequence alignment of the Nup98 GLEBS motif. The overall sequence conservation at each position is shaded in a color gradient from yellow (40% similarity) to red (100% identity) using the Blosum62 weighting algorithm (40). The numbering of the residues and the secondary structure are according to human Nup98. The secondary structure is indicated above the sequence as green arrows (β-strands), blue rectangles (α-helices), gray lines (coil regions), and gray dots (disordered residues). Dots below the sequence indicate residues involved in Rae1 binding (magenta and green). Residues that are shown in B are highlighted with magenta dots. Asterisks indicate the positions of the invariant Glu201 and Glu202 of the tandem glutamate element. (B) Details of the interaction between Rae1 and the Nup98 GLEBS motif. The ribbon representation is colored according to Fig. 1_B_. The inset illustrates the position of the tandem glutamate element and is expanded on the right. (C) Analysis of the interaction between Rae1 and GST-Nup98 GLEBS fragments and mutants. The C-terminal arm of the Nup98 GLEBS (GLEBS-C) is necessary and sufficient for Rae1 binding. A double mutant in Nup98 GLEBS, E201K/E202K, abolishes the Rae1-Nup98GLEBS interaction.
Fig. 3.
Structural comparison between the Rae1•Nup98 and Bub3•Mad3 complexes. The superposition of the Rae1 and Bub3 β-propeller domains is shown in the right panel. Bub3 and Mad3 are colored in gray and pink, respectively. The 5D6A interblade connectors and 7BC loops of the Rae1 and Bub3 β-propellers are indicated in different shades of green and yellow, respectively. A 90° rotated view is shown in the lower panel.
Fig. 5.
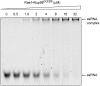
Rae1•Nup98GLEBS binds RNA in vitro. An electrophoretic mobility shift assay was carried out, with a constant amount of degenerate 10-mer RNA oligonucleotide (2 μM) and increasing concentrations of the Rae1•Nup98GLEBS complex, as indicated. The RNA oligonucleotide was visualized with SYBR Gold nucleic acid gel stain. The estimated dissociation constant of the interaction is in the low micromolar range.
Fig. 6.
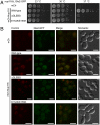
In vivo analysis of the Gle2-Nup116GLEBS interaction. (A) Yeast growth assay performed using _nup116_Δ, Gle2-GFP cells transformed with the indicated mCherry (mCh)-Nup116 constructs. Ten-fold serial dilutions were spotted on SD-LEU plates and grown for 2–3 days at the indicated temperatures. (B) In vivo localization of Gle2-GFP and mCh-Nup116 carried out at 30 °C. In the presence of full-length mCh-Nup116, Gle2-GFP is enriched at the nuclear envelope. The ΔGLEBS and the tandem glutamate element (E154K/E155K) Nup116 mutants result in a dispersed staining of Gle2-GFP throughout the cell with no enrichment at the nuclear envelope. (Scale bar: 5 μm.)
Similar articles
- Vesiculoviral matrix (M) protein occupies nucleic acid binding site at nucleoporin pair (Rae1 • Nup98).
Quan B, Seo HS, Blobel G, Ren Y. Quan B, et al. Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9127-32. doi: 10.1073/pnas.1409076111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927547 Free PMC article. - RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains.
Pritchard CE, Fornerod M, Kasper LH, van Deursen JM. Pritchard CE, et al. J Cell Biol. 1999 Apr 19;145(2):237-54. doi: 10.1083/jcb.145.2.237. J Cell Biol. 1999. PMID: 10209021 Free PMC article. - Molecular mechanism underlying selective inhibition of mRNA nuclear export by herpesvirus protein ORF10.
Feng H, Tian H, Wang Y, Zhang Q, Lin N, Liu S, Yu Y, Deng H, Gao P. Feng H, et al. Proc Natl Acad Sci U S A. 2020 Oct 27;117(43):26719-26727. doi: 10.1073/pnas.2007774117. Epub 2020 Oct 8. Proc Natl Acad Sci U S A. 2020. PMID: 33033226 Free PMC article. - Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export.
Folkmann AW, Noble KN, Cole CN, Wente SR. Folkmann AW, et al. Nucleus. 2011 Nov-Dec;2(6):540-8. doi: 10.4161/nucl.2.6.17881. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064466 Free PMC article. Review. - A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm.
Izaurralde E. Izaurralde E. Eur J Cell Biol. 2002 Nov;81(11):577-84. doi: 10.1078/0171-9335-00273. Eur J Cell Biol. 2002. PMID: 12498157 Review.
Cited by
- Post-translational O-GlcNAcylation is essential for nuclear pore integrity and maintenance of the pore selectivity filter.
Zhu Y, Liu TW, Madden Z, Yuzwa SA, Murray K, Cecioni S, Zachara N, Vocadlo DJ. Zhu Y, et al. J Mol Cell Biol. 2016 Feb;8(1):2-16. doi: 10.1093/jmcb/mjv033. Epub 2015 Jun 1. J Mol Cell Biol. 2016. PMID: 26031751 Free PMC article. - Molecular interactions of FG nucleoporin repeats at high resolution.
Ibáñez de Opakua A, Geraets JA, Frieg B, Dienemann C, Savastano A, Rankovic M, Cima-Omori MS, Schröder GF, Zweckstetter M. Ibáñez de Opakua A, et al. Nat Chem. 2022 Nov;14(11):1278-1285. doi: 10.1038/s41557-022-01035-7. Epub 2022 Sep 22. Nat Chem. 2022. PMID: 36138110 Free PMC article. - Regulation of mRNA trafficking by nuclear pore complexes.
Bonnet A, Palancade B. Bonnet A, et al. Genes (Basel). 2014 Sep 2;5(3):767-91. doi: 10.3390/genes5030767. Genes (Basel). 2014. PMID: 25184662 Free PMC article. Review. - Vesiculoviral matrix (M) protein occupies nucleic acid binding site at nucleoporin pair (Rae1 • Nup98).
Quan B, Seo HS, Blobel G, Ren Y. Quan B, et al. Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9127-32. doi: 10.1073/pnas.1409076111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927547 Free PMC article. - Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression.
Terlecki-Zaniewicz S, Humer T, Eder T, Schmoellerl J, Heyes E, Manhart G, Kuchynka N, Parapatics K, Liberante FG, Müller AC, Tomazou EM, Grebien F. Terlecki-Zaniewicz S, et al. Nat Struct Mol Biol. 2021 Feb;28(2):190-201. doi: 10.1038/s41594-020-00550-w. Epub 2021 Jan 21. Nat Struct Mol Biol. 2021. PMID: 33479542 Free PMC article.
References
- Moore MJ. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. - PubMed
- Stewart M. Ratcheting mRNA out of the nucleus. Mol Cell. 2007;25:327–330. - PubMed
- Brown JA, et al. A mutation in the Schizosaccharomyces pombe rae1 gene causes defects in poly(A)+ RNA export and in the cytoskeleton. J Biol Chem. 1995;270:7411–7419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases