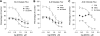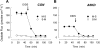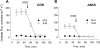Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1 - PubMed (original) (raw)
Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1
Robert W Freel et al. Am J Physiol Gastrointest Liver Physiol. 2009 Nov.
Abstract
The purpose of this investigation was to quantitate the contribution of the anion exchanger PAT-1 (putative anion transporter-1), encoded by SLC26A6, to oxalate transport in a model intestinal epithelium and to discern some characteristics of this exchanger expressed in its native environment. Control (Con) Caco-2 BBe1 monolayers, 6-8 days postseeding, were compared with those transfected with a small interfering RNA targeted to SLC26A6 (A6KD). Radiotracer and Ussing chamber techniques were used to determine the transepithelial unidirectional fluxes of Ox(2-), Cl(-), and SO(4)(2-) whereas fluorometric/BCECF measurements of intracellular pH were used to assess HCO(3)(-) exchange. PAT-1 was functionally targeted to the apical membrane, and SLC26A6 knockdown reduced PAT-1 protein (>60%) and mRNA (>75%) expression in A6KD. No net flux of Ox(2-), Cl(-), or SO(4)(2-) was detected in Con or A6KD monolayers, yet the unidirectional fluxes in A6KD were reduced 50, 35, and 15%, respectively. Cl(-)-dependent HCO(3)(-) efflux from A6KD was reduced 50% compared with Con. The difference between Con and A6KD properties represents that mediated solely by PAT-1, and by this approach we found that PAT-1-mediated oxalate influx and efflux are inhibited equally by mucosal DIDS (EC(50) approximately 5 microM) and that mucosal Cl(-) inhibits oxalate uptake with an EC(50) < 20 mM. Transepithelial Cl(-) gradients supported large, DIDS-sensitive net absorptive or secretory fluxes of oxalate in a direction opposite that of the imposed Cl(-) gradient. The overall symmetry of PAT-1-mediated oxalate exchange suggests that vectorial oxalate transport observed in vivo is principally dependent on the magnitude and direction of counterion gradients.
Figures
Fig. 1.
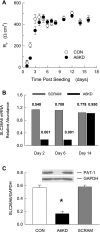
Validation of SLC26A6 silencing using small interfering RNA (siRNA) techniques in Caco-2 monolayers. A: development of transepithelial resistance (RT) is presented for Con and A6KD monolayers seeded at 4 × 105 cells/cm2. By the fourth day postseeding there were no significant differences in RT measured between control (Con) and SLC26A6 knockdown (A6KD) monolayers (n = 9). B: persistence of siRNA-induced depression of SLC26A6 mRNA. The expression of SLC26A6 mRNA relative to GAPDH mRNA from Con, scrambled siRNA (Scram), and A6KD monolayers were quantitated by real-time RT-PCR (n = 6). The relative change in expression for Scram and A6KD were computed by the ΔΔCT method as provided in the Relative Expression Software Tool spreadsheet [REST384 (20)] to statistically evaluate the fold changes in gene expression. The numbers above each bar are the probability values generated by the REST analysis for a comparison of a sample with its respective controls. Scrambled siRNA did not significantly affect the expression of SLC26A6 mRNA, whereas siRNA for SLC26A6 produced significant reduction in SLC26A6 message at days 2 and 6. C: relative abundance of SLC26A6 to GAPDH in Con, Scram, and A6KD monolayers 6 days postseeding. Error bars represent ±1 SE (n = 4); *A6KD monolayers are significantly different from Con and Scram (in a 1-way ANOVA). Inset shows an immunoblot for SLC26A6 and the reference protein GAPDH in the same membrane.
Fig. 2.
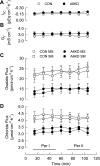
Temporal stability of transepithelial electrical properties and unidirectional anion fluxes across Con and A6KD Caco-2 monolayers 6–8 days postseeding in standard bicarbonate buffer. All open symbols in this figure represent Con monolayers and solid symbols signify A6KD monolayers. In the flux panels (C and D), circles represent mucosal-to-serosal flux (_J_MS) and squares serosal-to-mucosal flux (_J_SM). Short-circuit current (_I_sc) is presented in A and transepithelial conductance (GT) is shown in B; these parameters were obtained during the measurements of oxalate flux. C: unidirectional oxalate fluxes in Con (n = 9) and A6KD (n = 9) monolayers. D: comparison of unidirectional chloride fluxes in Con (n = 8) and A6KD (n = 8) monolayers. Horizontal bars at the bottom of the figure define the intervals that make up periods I and II (Per I and Per II), which are used throughout for statistical comparisons. Error bars represent ±1 SE.
Fig. 3.
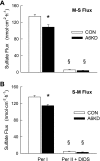
Unidirectional sulfate fluxes across control (open bars) and A6KD (solid bars) Caco-2 monolayers. Mucosal-to-serosal fluxes (M-S; A) and serosal-to-mucosal fluxes (S-M; B) were made in symmetrical bicarbonate-free buffers under short-circuit conditions. Per I represents measurements in control conditions and Per II represents fluxes after the addition of 100 μM DIDS to the mucosal compartment. In Per I, GT was 2.57 ± 0.16 for Con and 2.42 ± 0.32 for A6KD monolayers. *Significant difference between Con and A6KD monolayers; §difference from Per I. Error bars represent ±1 SE and n = 6 for each condition.
Fig. 4.
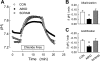
A6KD reduces the rate of Cl−-dependent acidification (a measure of Cl−-HCO3− exchange) in Caco-2 monolayers compared with Con and Scram monolayers. Intracellular pH was measured in cells superfused with standard HCO3−/CO2 buffer with and without Cl−. As shown in A, removal of Cl− resulted in an increase in intracellular pH (pHi) for all monolayers whereas readmission of Cl− prompted a return of pHi to initial values. The average initial rates (δpHi/min) of alkalinization on Cl− removal and acidification on Cl− readmission are presented in B and C, respectively. In A6KD monolayers, Cl−-HCO3− exchange is significantly reduced to about half that of Con or Scram monolayers as judged by a 1-way ANOVA. Typical recordings of at least 7 experiments.
Fig. 5.
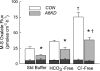
Symmetrical replacement of bicarbonate or chloride increases _J_MSOx in short-circuited Caco-2 monolayers 6–8 days postseeding. Open bars, Con monolayers; shaded bars, cells transfected with SLC26A6 siRNA (A6KD). Stilbene sensitivity was assessed in Per II by the addition of 100 μM DIDS to the mucosal compartment, and the residual, DIDS-insensitive components are indicated by the dark portion at the base of each bar. In standard buffer (Std Buffer, n = 6), GT was 3.07 ± 0.12 for Con and 3.49 ± 0.25 for A6KD monolayers and in HCO3−-free buffers (n = 10) GT was not significantly changed. In Cl−-free buffer (n = 6), GT was 1.43 ± 0.06 for Con and 1.40 ± 0.08 for A6KD monolayers. *Significant difference between Con and A6KD in a given buffer; †significant difference from the appropriate standard control buffer as judged by a 1-way ANOVA. Error bars represent ±1 SE.
Fig. 6.
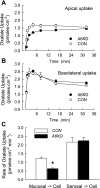
Time course of oxalate accumulation across the apical membrane (A) and basolateral membrane (B) for Con and A6KD monolayers in bicarbonate-free buffers. Apical uptake was determined with a Cl−-HCO3−-free mucosal buffer and a HCO3−-free serosal buffer, whereas the basolateral uptake measurements were made with the buffer compositions reversed. Each point represents the mean of 6–8 measurements and the error bars represent ±1 SE. *Significant difference between Con and A6KD.
Fig. 7.
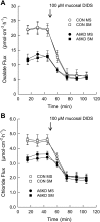
DIDS inhibition of unidirectional anion fluxes across Caco-2 monolayers in standard bicarbonate buffer. Open symbols in this figure represent Con monolayers, solid symbols signify A6KD monolayers; circles represent _J_MS (MS) and squares _J_SM (SM). A: time course of mucosal DIDS (100 μM) inhibition of [14C]oxalate transport across short-circuited Con monolayers (n = 7) and A6KD (n = 9) monolayers. B: time course for 36Cl− fluxes measured across Con (n = 7) and A6KD (n = 7) Caco-2 monolayers. Current and conductance were not significantly altered by mucosal DIDS. Error bars represent ±1 SE.
Fig. 8.
Concentration dependence of mucosal DIDS inhibition of oxalate and chloride transport across Con (○, n = 6) and A6KD (•, n = 6) Caco-2 monolayers 6–8 days postseeding in standard bicarbonate buffer. A: effect of DIDS on _J_MSOx. The percent inhibition was calculated relative to the mean _J_MSOx measured in Per I of all controls used in this series (25.6 ± 1.3 pmol·cm−2·h−1, n = 42). The GT for Con in Per I (2.89 ± 0.08 mS·cm−2) was not significantly different from GT in A6KD monolayers (2.78 ± 0.09 mS·cm−2). In B, the effect of DIDS on _J_SMOx is shown. Again, the percent inhibition was calculated relative to the mean _J_SMOx measured in Per I of all controls used in this series (26.8 ± 1.9 pmol·cm−2·h−1, n = 42). Conductances in Per I were similar to those presented in A. C: concentration dependence of mucosal DIDS inhibition of _J_MSCl in Con (n = 5) and A6KD (n = 5) monolayers. The percent inhibition was calculated relative to the mean _J_MSCl measured in Per I of all controls used in this series (4.26 ± 0.13 pmol·cm−2·h−1, n = 30). The GT for Con in Per I (2.65 ± 0.43 mS·cm−2) was not significantly different from that of A6KD monolayers (2.46 ± 0.11 mS·cm−2). In each panel the dashed line through the shaded squares represents the dose-response curve for SLC26A6 exchanger alone (i.e., the difference between Con and A6KD monolayers). Error bars represent ±1 SE.
Fig. 9.
Chloride dependence of _J_MSOx in Con (○) and A6KD (•) Caco-2 monolayers 6–8 days postseeding in bicarbonate-free buffers. Mucosal chloride was varied by replacing it with gluconate, and the serosal compartment contained normal bicarbonate-free buffer. There were no significant differences in GT between Con and A6KD monolayers bathed by the same buffers. The mean fluxes from Per I are presented (n = 6) and error bars represent ±1 SE. Inset represents the degree of inhibition of SLC26A6-mediated _J_MSOx, which was calculated as the differences between oxalate fluxes in Con and A6KD monolayers and presented as a percentage of the maximum flux measured in 0 Cl−. The solid line in the inset was generated by a curve fit to the data points using 1 site saturation binding, which yielded an EC50 = 18.7 ± 3.3 mM.
Fig. 10.
Transmural (serosal-to-mucosal) chloride gradients generate large, persistent, DIDS-sensitive net absorptive fluxes of oxalate in Caco-2 monolayers 6–8 days postseeding. Mucosal buffers were nominally Cl− and HCO3− free, and the serosal buffers were HCO3− free. Results from Con monolayers are presented in A and A6KD monolayers in B. Error bars represent ±1 SE and n = 6 and may be contained within the limits of the symbols. Short-circuit current in Per I Con was not significantly different from that of A6KD monolayers (−0.52 ± 0.10 and −0.63 ± 0.07 μeq·cm−2·h−1, respectively) nor were the conductances different (2.03 ± 0.13 and 2.23 ± 0.11 mS·cm−2, respectively).
Fig. 11.
Mucosal-to-serosal chloride gradients generate a large, DIDS-sensitive net secretion of oxalate in Con (A) and A6KD (B) monolayers of Caco-2 cells 6–8 days postseeding. Serosal buffers were nominally Cl− and HCO3− free whereas the mucosal buffers were HCO3− free. Error bars represent ±1 SE (n = 5) and may be contained within the limits of the symbols. In Per I the short-circuit current of Con monolayers was not significantly different from that of A6KD monolayers (0.33 ± 0.03 and 0.28 ± 0.03 μeq·cm−2·h−1, respectively); nor were the conductances different between the 2 groups (2.66 ± 0.33 and 2.63 ± 0.41 mS·cm−2, respectively).
Similar articles
- PAT-1 (Slc26a6) is the predominant apical membrane Cl-/HCO3- exchanger in the upper villous epithelium of the murine duodenum.
Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. Simpson JE, et al. Am J Physiol Gastrointest Liver Physiol. 2007 Apr;292(4):G1079-88. doi: 10.1152/ajpgi.00354.2006. Epub 2006 Dec 14. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17170027 - Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity.
Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Chernova MN, et al. J Biol Chem. 2005 Mar 4;280(9):8564-80. doi: 10.1074/jbc.M411703200. Epub 2004 Nov 17. J Biol Chem. 2005. PMID: 15548529 - The anion exchanger PAT-1 (Slc26a6) does not participate in oxalate or chloride transport by mouse large intestine.
Whittamore JM, Hatch M. Whittamore JM, et al. Pflugers Arch. 2021 Jan;473(1):95-106. doi: 10.1007/s00424-020-02495-x. Epub 2020 Nov 17. Pflugers Arch. 2021. PMID: 33205229 Free PMC article. - Role of SLC26A6-mediated Cl⁻-oxalate exchange in renal physiology and pathophysiology.
Aronson PS. Aronson PS. J Nephrol. 2010 Nov-Dec;23 Suppl 16:S158-64. J Nephrol. 2010. PMID: 21170874 Review. - Essential roles of CFEX-mediated Cl(-)-oxalate exchange in proximal tubule NaCl transport and prevention of urolithiasis.
Aronson PS. Aronson PS. Kidney Int. 2006 Oct;70(7):1207-13. doi: 10.1038/sj.ki.5001741. Epub 2006 Aug 2. Kidney Int. 2006. PMID: 16883319 Review.
Cited by
- The SLC26 gene family of anion transporters and channels.
Alper SL, Sharma AK. Alper SL, et al. Mol Aspects Med. 2013 Apr-Jun;34(2-3):494-515. doi: 10.1016/j.mam.2012.07.009. Mol Aspects Med. 2013. PMID: 23506885 Free PMC article. Review. - Sulfate secretion and chloride absorption are mediated by the anion exchanger DRA (Slc26a3) in the mouse cecum.
Whittamore JM, Freel RW, Hatch M. Whittamore JM, et al. Am J Physiol Gastrointest Liver Physiol. 2013 Jul 15;305(2):G172-84. doi: 10.1152/ajpgi.00084.2013. Epub 2013 May 9. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23660504 Free PMC article. - Regulated transport of sulfate and oxalate by SLC26A2/DTDST.
Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL. Heneghan JF, et al. Am J Physiol Cell Physiol. 2010 Jun;298(6):C1363-75. doi: 10.1152/ajpcell.00004.2010. Epub 2010 Mar 10. Am J Physiol Cell Physiol. 2010. PMID: 20219950 Free PMC article. - Activation of the PKA signaling pathway stimulates oxalate transport by human intestinal Caco2-BBE cells.
Arvans D, Alshaikh A, Bashir M, Weber C, Hassan H. Arvans D, et al. Am J Physiol Cell Physiol. 2020 Feb 1;318(2):C372-C379. doi: 10.1152/ajpcell.00135.2019. Epub 2019 Dec 11. Am J Physiol Cell Physiol. 2020. PMID: 31825656 Free PMC article. - SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization.
Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL. Stewart AK, et al. Am J Physiol Cell Physiol. 2011 Aug;301(2):C289-303. doi: 10.1152/ajpcell.00089.2011. Epub 2011 May 18. Am J Physiol Cell Physiol. 2011. PMID: 21593449 Free PMC article.
References
- Alper SL, Stewart AK, Chernova MN, Zolotarev AS, Clark JS, Vandorpe DH. Anion exchangers in flux: functional differences between human and mouse SLC26A6 polypeptides. Novartis Found Symp 273: 107–119; discussion 119–125, 261–104, 2006 - PubMed
- Alrefai WA, Tyagi S, Mansour F, Saksena S, Syed I, Ramaswamy K, Dudeja PK. Sulfate and chloride transport in Caco-2 cells: differential regulation by thyroxine and the possible role of DRA gene. Am J Physiol Gastrointest Liver Physiol 280: G603–G613, 2001 - PubMed
- Aronson PS. Role of SLC26-mediated Cl−/base exchange in proximal tubule NaCl transport. Novartis Found Symp 273: 148–158; discussion 158–163, 261–144, 2006 - PubMed
- Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844–C856, 1988 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials