Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis - PubMed (original) (raw)
Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis
Ludmila Khailova et al. Am J Physiol Gastrointest Liver Physiol. 2009 Nov.
Abstract
Neonatal necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in premature infants. Oral administration of probiotics has been suggested as a promising strategy for prevention of NEC. However, little is known about the mechanism(s) of probiotic-mediated protection against NEC. The aim of this study was to evaluate the effects of Bifidobacterium bifidum treatment on development of NEC, cytokine regulation, and intestinal integrity in a rat model of NEC. Premature rats were divided into three groups: dam fed (DF), hand fed with formula (NEC), or hand fed with formula supplemented with 5 x 10(6) CFU B. bifidum per day (B. bifidum). All groups were exposed to asphyxia and cold stress to develop NEC. Intestinal injury, mucin and trefoil factor 3 (Tff3) production, cytokine levels, and composition of tight junction (TJ) and adherens junction (AJ) proteins were evaluated in the terminal ileum. B. bifidum decreased the incidence of NEC from 57 to 17%. Increased levels of IL-6, mucin-3, and Tff3 in the ileum of NEC rats was normalized in B. bifidum treated rats. Reduced mucin-2 production in the NEC rats was not affected by B. bifidum. Administration of B. bifidum normalized the expression and localization of TJ and AJ proteins in the ileum compared with animals with NEC. In conclusion, administration of B. bifidum protects against NEC in the neonatal rat model. This protective effect is associated with reduction of inflammatory reaction in the ileum, regulation of main components of mucus layer, and improvement of intestinal integrity.
Figures
Fig. 1.
Histological scoring of the terminal ileum of neonatal rats. The ileum stained with hematoxylin and eosin showing representative sections for each morphological grading score. A: necrotizing enterocolitis (NEC) score 0, normal ileum. B: NEC score 1, mild damage with slight submucosal and/or lamina propria separation. C: NEC score 2, moderate to severe separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers, partial villous sloughing. D: NEC score 3, severe separation of submucosa and/or lamina propria, region villous sloughing and initial villus necrosis. E: NEC score 4, necrosis and loss of villi structure and/or transmural necrosis. Magnification: ×200.
Fig. 2.
Severity and incidence of NEC in neonatal rat model. A: histological NEC score in the dam-fed (DF; n = 16), NEC (n = 28), and Bifidobacterium bifidum (n = 29) groups are shown. Ileal damage was assessed by the histological scoring system described in Fig. 1. Bars indicate median. B: incidence of NEC in the neonatal rat model of NEC. Animals with scores ≥2 are considered NEC positive; animals with ileal damage <2 do not have NEC. #P ≤ 0.01 vs. NEC, χ2 analysis.
Fig. 3.
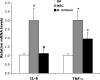
IL-6 and TNF-α mRNA levels in neonatal rat ileum. The mean steady-state mRNA level for the DF group was assigned a value of 1.0, and mean mRNA levels for the NEC and B. bifidum groups were determined relative to this number. Values are means ± SE; n = 10–12 animals/experimental group. *P ≤ 0.01 vs. DF. #P ≤ 0.01 vs. NEC.
Fig. 4.
Muc2- and Tff3-positive cells in the ileum of neonatal rats. A: Muc2-stained representative slides from DF, NEC, and B. bifidum groups are shown. Magnification: ×400. Enumeration of Muc2-positive cells in neonatal rat ileum is shown in the graph (n = 9 animals/experimental group). Data are expressed as mean Muc2-positive cells/100 epithelial cells ± SE. *P ≤ 0.01 vs. DF. B: Tff3-stained representative slides from DF, NEC, and B. bifidum groups are shown. Magnification: ×400. Enumeration of TFF3-positive cells in neonatal rat ileum is shown in the graph (n = 9 animals/experimental group). Numbers are expressed as mean Tff3-positive cells/100 epithelial cells ± SE. *P ≤ 0.01 vs. DF, #P ≤ 0.01 vs. DF.
Fig. 5.
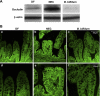
Effect of oral administration of B. bifidum on expression and localization of occludin. A: representative 65-kDa occludin bands from Western blot analyses are shown for the DF, NEC, and B. bifidum groups. B: representative slides from DF, NEC, and B. bifidum groups evaluated by confocal laser scanning microscopy (n = 6 animals/experimental group). No signal was observed in negative control sections (not shown). Magnification: ×400 (a, b, c), ×800 (d, e, f).
Fig. 6.
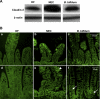
Effect of oral administration of B. bifidum on expression and localization of claudin-3. A: representative 22-kDa claudin-3 bands from Western blot analyses are shown for DF, NEC, and B. bifidum groups. B: representative slides from DF, NEC, and B. bifidum groups were evaluated by confocal laser scanning microscopy (n = 6 animals/experimental group). In the NEC group, claudin-3 signal was dispersed throughout the cytoplasm (e; arrowhead). In the B. bifidum group, claudin-3 signal was associated with enterocyte membrane (f; arrows). No signal was observed in negative control sections (not shown). Magnification: ×400 (a, b, c), ×800 (d, e, f).
Fig. 7.
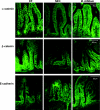
Localization of adherens junction proteins (α-catenin, β-catenin, and E-cadherin) evaluated by inverted fluorescence microscopy. Representative slides from DF, NEC, and B. bifidum groups are shown (n = 6 animals/experimental group). No signal was observed in negative control sections (not shown). Magnification: ×400.
Similar articles
- Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis.
Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Khailova L, et al. Am J Physiol Gastrointest Liver Physiol. 2010 Nov;299(5):G1118-27. doi: 10.1152/ajpgi.00131.2010. Epub 2010 Aug 12. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20705904 Free PMC article. - Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model.
Ling X, Linglong P, Weixia D, Hong W. Ling X, et al. PLoS One. 2016 Aug 23;11(8):e0161635. doi: 10.1371/journal.pone.0161635. eCollection 2016. PLoS One. 2016. PMID: 27551722 Free PMC article. - Pomegranate seed oil reduces intestinal damage in a rat model of necrotizing enterocolitis.
Coursodon-Boyiddle CF, Snarrenberg CL, Adkins-Rieck CK, Bassaganya-Riera J, Hontecillas R, Lawrence P, Brenna JT, Jouni ZE, Dvorak B. Coursodon-Boyiddle CF, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Sep 15;303(6):G744-51. doi: 10.1152/ajpgi.00248.2012. Epub 2012 Jul 19. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22821948 Free PMC article. - Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis.
Martin CR, Walker WA. Martin CR, et al. Semin Perinatol. 2008 Apr;32(2):127-37. doi: 10.1053/j.semperi.2008.01.006. Semin Perinatol. 2008. PMID: 18346537 Review. - Probiotics in the prevention of necrotizing enterocolitis.
Ganguli K, Walker WA. Ganguli K, et al. J Clin Gastroenterol. 2011 Nov;45 Suppl:S133-8. doi: 10.1097/MCG.0b013e318228b799. J Clin Gastroenterol. 2011. PMID: 21992952 Review.
Cited by
- Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis.
Maynard AA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A, Dvorak B. Maynard AA, et al. Am J Physiol Gastrointest Liver Physiol. 2010 Sep;299(3):G614-22. doi: 10.1152/ajpgi.00076.2010. Epub 2010 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20539009 Free PMC article. - Biotechnological Processes Simulating the Natural Fermentation Process of Bee Bread and Therapeutic Properties-An Overview.
Barta DG, Cornea-Cipcigan M, Margaoan R, Vodnar DC. Barta DG, et al. Front Nutr. 2022 Apr 27;9:871896. doi: 10.3389/fnut.2022.871896. eCollection 2022. Front Nutr. 2022. PMID: 35571893 Free PMC article. Review. - The inhibition of enterocyte proliferation by lithocholic acid exacerbates necrotizing enterocolitis through downregulating the Wnt/β-catenin signalling pathway.
Feng Z, Jia C, Lin X, Hao H, Li S, Li F, Cui Q, Chen Y, Wu F, Xiao X. Feng Z, et al. Cell Prolif. 2022 May;55(5):e13228. doi: 10.1111/cpr.13228. Epub 2022 Apr 20. Cell Prolif. 2022. PMID: 35441471 Free PMC article. - Impact of probiotics on necrotizing enterocolitis.
Underwood MA. Underwood MA. Semin Perinatol. 2017 Feb;41(1):41-51. doi: 10.1053/j.semperi.2016.09.017. Epub 2016 Nov 8. Semin Perinatol. 2017. PMID: 27836423 Free PMC article. Review. - Effect of bifidobacterium on defensin-5 expression in intestinal injury of preweaning rats.
Wang W, Yang SF, Ren LH, Zhang XX, Yu SL. Wang W, et al. World J Gastroenterol. 2015 Mar 7;21(9):2638-44. doi: 10.3748/wjg.v21.i9.2638. World J Gastroenterol. 2015. PMID: 25759531 Free PMC article.
References
- Amit-Romach E, Reifen R, Uni Z. Mucosal function in rat jejunum and ileum is altered by induction of colitis. Int J Mol Med 18: 721–727, 2006 - PubMed
- Amit-Romach E, Uni Z, Cheled S, Berkovich Z, Reifen R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J Nutr Biochem 20: 70–77, 2009 - PubMed
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005 - PubMed
- Blakey JL, Lubitz L, Campbell NT, Gillam GL, Bishop RF, Barnes GL. Enteric colonization in sporadic neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 4: 591–595, 1985 - PubMed
- Blaschuk OW, Rowlands TM. Plasma membrane components of adherens junctions (Review). Mol Membr Biol 19: 75–80, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous