Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas - PubMed (original) (raw)
Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas
Sung-Suk Chae et al. Clin Cancer Res. 2010.
Abstract
Purpose: In brain tumors, cerebral edema is a significant source of morbidity and mortality. Recent studies have shown that inhibition of vascular endothelial growth factor (VEGF) signaling induces transient vascular normalization and reduces cerebral edema, resulting in a modest survival benefit in glioblastoma patients. During anti-VEGF treatment, circulating levels of angiopoietin (Ang)-2 remained high after an initial minor reduction. It is not known, however, whether Ang-2 can modulate anti-VEGF treatment of glioblastoma. Here, we used an orthotopic glioma model to test the hypothesis that Ang-2 is an additional target for improving the efficacy of current anti-VEGF therapies in glioma patients.
Experimental design: To recapitulate high levels of Ang-2 in glioblastoma patients during anti-VEGF treatment, Ang-2 was ectopically expressed in U87 glioma cells. Animal survival and tumor growth were assessed to determine the effects of Ang-2 and anti-VEGF receptor 2 (VEGFR2) treatment. We also monitored morphologic and functional vascular changes using multiphoton laser scanning microscopy and immunohistochemistry.
Results: Ectopic expression of Ang-2 had no effect on vascular permeability, tumor growth, or survival, although it resulted in higher vascular density, with dilated vessels and reduced mural cell coverage. On the other hand, when combined with anti-VEGFR2 treatment, Ang-2 destabilized vessels without affecting vessel regression and compromised the survival benefit of VEGFR2 inhibition by increasing vascular permeability. VEGFR2 inhibition normalized tumor vasculature whereas ectopic expression of Ang-2 diminished the beneficial effects of VEGFR2 blockade by inhibiting vessel normalization.
Conclusion: Cancer treatment regimens combining anti-VEGF and anti-Ang-2 agents may be an effective strategy to improve the efficacy of current anti-VEGF therapies.
Copyright 2010 AACR.
Figures
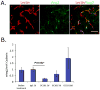
Figure 1. Expression of Ang-2 during VEGFR2 inhibition
A. Untreated tumor tissue sections were stained for ECs (Red) and Ang-2 (Green). Ang-2 was dominantly expressed by ECs. Scale bar, 50 μm. B. Ang-2 and VE-Cadherin transcript levels were semi-quantitatively determined by real-time PCR. Ang-2 expression was transiently reduced by DC101 treatment. N=5.
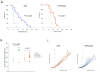
Figure 2. Effect of Ang-2 and VEGFR2 inhibition on tumor growth and survival
A. Tumor bearing mice were treated with IgG (dotted line) or DC101 (solid line) and followed for their survival. DC101 treatment significantly extended survival of mice, and Ang-2 compromised the survival benefit of DC101. B. DC101 treated mice (filled circle) survived with significantly larger tumors compared to untreated mice (open circle), but not Ang-2 tumor bearing mice. C. Neither Ang-2 nor DC101 treatment had an effect on tumor growth. Purple and brown lines indicate IgG and DC101 treatments in control tumors. Light blue and blue lines indicate IgG and DC101 treatments in U87-Ang-2 tumors. Each line represents an individual mouse.
Figure 3. Permeability changes in tumor vasculature by Ang-2 and VEGFR2 inhibition
A. DC101 treatment decreased vascular permeability temporarily in control tumors. N= 8 (Ig G) and 9 (DC101). B. Ang-2 interfered with the effect of DC101 on vascular permeability. DC101 treatment did not reduce the vascular permeability in U87-Ang-2 tumors. N=7 (IgG) and 8 (DC101). C. MR imaging was performed before (Day 0) and 2 day (Day 2) after treatment with IgG or DC101. Representative T2-weighted images were shown. Arrows indicate the tumors grown in cranial windows. Scale bar, 2mm. D. DC101 treatment was more effective in reducing tumor edema in U87 tumors than in U87Ang-2 tumors. N= 7.
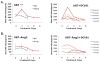
Figure 4. Effect of Ang-2 and VEGFR2 blockage on RBC velocity
A. Average RBC velocity was temporally increased by DC101 treatment in control tumors. The regulation kinetics of RBC velocity correlated with kinetics of vascular permeability and Ang-2 expression. B. DC101 treatment had no effect on RBC velocity in U87-Ang-2 tumors. Left panels show average velocity data and right panels show the data from individual animals. N=5.
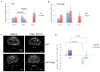
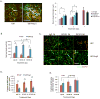
Figure 5. Effect of Ang-2 and VEGFR2 blockage on tumor blood vessels
A. Tumor vasculature was imaged using MPLSM after the injection of rhodamine conjugated dextran and DiD labeled RBC. Green color shows the perfused vessels with rhodamine conjugated vessel and Red color shows labeled RBCs. Vessels were dilated in U87-Ang-2 tumors compared to control tumor vasculature. DC101 treatment had no significant effect on vessel diameter. Mean vessel diameters were determined from Z stack images collected using MPLSM. Scale bar, 200 μm. B. Ang-2 increased total vessel length. DC101 treatment was equally effective in vessel trimming in both tumors. C. Immunohistochemical analysis was performed and images were collected using confocal laser scanning microscopy. The representative images of blood vessels and pericyte coverage after VEGFR-2 inhibition are shown. Blood vessels and pericytes from the frozen sections were stained for lectin (Green) and αSMA (Red), respectively. D. Ang-2 decreased α-SMA positive fraction in vessels. DC101 treatment increased α-SMA positive cell coverage in control tumors but not in U87Ang-2 tumors. E. Ang-2 had no effect on NG-2 positive fraction while interfering with the increase in NG-2 positive vessel fraction by DC101 treatment. DC101 treatment increased α-SMA positive cell coverage only in control tumors. N=8 (Day 0 and 2) and 4 (Day 8).
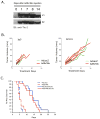
Figure 6. Effects of Tie-2 inhibition on tumor growth and mouse survival with VEGFR2 inhibition
A. Single intravenous administration of ExTek adenovirus maintained the sTie-2 expression for more than 2 weeks. #; animal number. B. sTie-2 had no effect on tumor growth in IgG (left panel) or DC101 (right panel) treated mice. N=6. C. sTie-2 had no significant effect on the survival benefit of VEGFR2 inhibition.
Similar articles
- Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model.
Whitehurst B, Flister MJ, Bagaitkar J, Volk L, Bivens CM, Pickett B, Castro-Rivera E, Brekken RA, Gerard RD, Ran S. Whitehurst B, et al. Int J Cancer. 2007 Nov 15;121(10):2181-91. doi: 10.1002/ijc.22937. Int J Cancer. 2007. PMID: 17597103 - Efficacy of a Bispecific Antibody Co-Targeting VEGFA and Ang-2 in Combination with Chemotherapy in a Chemoresistant Colorectal Carcinoma Xenograft Model.
Mueller T, Freystein J, Lucas H, Schmoll HJ. Mueller T, et al. Molecules. 2019 Aug 7;24(16):2865. doi: 10.3390/molecules24162865. Molecules. 2019. PMID: 31394786 Free PMC article. - Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth.
Cao Y, Sonveaux P, Liu S, Zhao Y, Mi J, Clary BM, Li CY, Kontos CD, Dewhirst MW. Cao Y, et al. Cancer Res. 2007 Apr 15;67(8):3835-44. doi: 10.1158/0008-5472.CAN-06-4056. Cancer Res. 2007. PMID: 17440098 - Autocrine VEGFR1 and VEGFR2 signaling promotes survival in human glioblastoma models in vitro and in vivo.
Szabo E, Schneider H, Seystahl K, Rushing EJ, Herting F, Weidner KM, Weller M. Szabo E, et al. Neuro Oncol. 2016 Sep;18(9):1242-52. doi: 10.1093/neuonc/now043. Epub 2016 Mar 23. Neuro Oncol. 2016. PMID: 27009237 Free PMC article. - Novel anti-angiogenic therapies for malignant gliomas.
Norden AD, Drappatz J, Wen PY. Norden AD, et al. Lancet Neurol. 2008 Dec;7(12):1152-60. doi: 10.1016/S1474-4422(08)70260-6. Lancet Neurol. 2008. PMID: 19007739 Review.
Cited by
- Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages.
Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, Datta M, Amoozgar Z, Seano G, Jung K, Kamoun WS, Vardam T, Snuderl M, Goveia J, Chatterjee S, Batista A, Muzikansky A, Leow CC, Xu L, Batchelor TT, Duda DG, Fukumura D, Jain RK. Peterson TE, et al. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4470-5. doi: 10.1073/pnas.1525349113. Epub 2016 Apr 4. Proc Natl Acad Sci U S A. 2016. PMID: 27044097 Free PMC article. - Disturbance in cerebral blood microcirculation and hypoxic-ischemic microenvironment are associated with the development of brain metastasis.
Roesler J, Spitzer D, Jia X, Aasen SN, Sommer K, Roller B, Olshausen N, Hebach NR, Albinger N, Ullrich E, Zhu L, Wang F, Macas J, Forster MT, Steinbach JP, Sevenich L, Devraj K, Thorsen F, Karreman MA, Plate KH, Reiss Y, Harter PN. Roesler J, et al. Neuro Oncol. 2024 Nov 4;26(11):2084-2099. doi: 10.1093/neuonc/noae094. Neuro Oncol. 2024. PMID: 38831719 Free PMC article. - Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease.
Goel S, Wong AH, Jain RK. Goel S, et al. Cold Spring Harb Perspect Med. 2012 Mar;2(3):a006486. doi: 10.1101/cshperspect.a006486. Cold Spring Harb Perspect Med. 2012. PMID: 22393532 Free PMC article. Review. - Leishmania major Infection-Induced VEGF-A/VEGFR-2 Signaling Promotes Lymphangiogenesis That Controls Disease.
Weinkopff T, Konradt C, Christian DA, Discher DE, Hunter CA, Scott P. Weinkopff T, et al. J Immunol. 2016 Sep 1;197(5):1823-31. doi: 10.4049/jimmunol.1600717. Epub 2016 Jul 29. J Immunol. 2016. PMID: 27474074 Free PMC article.
References
- Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–80. - PubMed
- Sie M, Wagemakers M, Molema G, Mooij JJ, de Bont ES, den Dunnen WF. The angiopoietin 1/angiopoietin 2 balance as a prognostic marker in primary glioblastoma multiforme. J Neurosurg. 2009;110:147–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA073479/CA/NCI NIH HHS/United States
- R01-CA96915/CA/NCI NIH HHS/United States
- R01 CA126642/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- P01-CA80124/CA/NCI NIH HHS/United States
- R01-CA85140/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- K25 AG029415/AG/NIA NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- T32-CA073479/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous