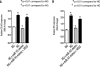Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions - PubMed (original) (raw)
Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions
Jianyin Long et al. J Biol Chem. 2010.
Abstract
Vascular endothelial growth factor (VEGF) is a dimeric glycoprotein that plays a crucial role in microvascular complications of diabetes, including diabetic nephropathy. However, the precise regulatory mechanisms governing VEGF expression in the diabetic milieu are still poorly understood. Here, we provide evidence that microRNA-93 (miR-93) regulates VEGF expression in experimental models of diabetes both in vitro and in vivo. Comparative microRNA expression profile arrays identified miR-93 as a signature microRNA in hyperglycemic conditions. We identified VEGF-A as a putative target of miR-93 in the kidney with a perfect complementarity between miR-93 and the 3'-untranslated region of vegfa in several species. When cotransfected with a luciferase reporter construct containing the mouse vegfa 3'-untranslated region, expression of miR-93 markedly decreased the luciferase activity. We showed that forced expression of miR-93 in cells abrogated VEGF protein secretion. Conversely, anti-miR-93 inhibitors increased VEGF release. Transfection of miR-93 also prevented the effect of high glucose on VEGF downstream targets. Using transgenic mice containing VEGF-LacZ bicistronic transcripts, we found that inhibition of glomerular miR-93 by peptide-conjugated morpholino oligomers elicited increased expression of VEGF. Our findings also indicate that high glucose decreases miR-93 expression by down-regulating the promoter of the host MCM7 gene. Taken together, our findings provide new insights into the role of miR-93 in VEGF signaling pathway and offer a potentially novel target in preventing the progression of diabetic nephropathy.
Figures
FIGURE 1.
Identification of miR-93 as a signature miRNA in high glucose (HG) conditions. Comparative microarray analysis indicated that 5 miRNAs were down-regulated within all samples, whereas 45 miRNAs were down-regulated in db/db glomeruli, 86 in high glucose-treated kidney microvascular endothelial cells, and 32 miRNAs in podocytes treated with high glucose (25 m
m
) for 24 h compared with normal glucose conditions.
FIGURE 2.
miR-93 is predicted to target vegfa gene. A, upper panel, miR-93 target site resides at nucleotides 162–168 (shown in the blue box) of the vegf 3′-UTR, is highly conserved in several species. Lower panel, sequence alignment of miR-93 with the mouse vegfa 3′-UTR is shown. B, miR-93 can potentially form a strong secondary structure with the target sequence of 3′-UTR of vegfa (predicted by RNA Hybrid).
FIGURE 3.
Expression pattern of miR-93 in vivo. A, expression of miR-93 in different tissues as detected by Northern blotting. U6 snRNA serves as a loading control. B, expression pattern of miR-93 in a db/m kidney tissue by in situ hybridization. Inset, higher power view. G, glomerulus; T, tubule. Images are representative of three independent experiments.
FIGURE 4.
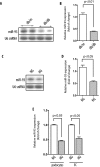
miR-93 is down-regulated in high glucose conditions. A, Northern blot analysis of representative results from glomerular miR-93 expression in two control db/m and two diabetic db/db mice. B, quantitative analysis of miR-93 expression. Mean values for miR-93 expression were generated by measuring the pixel intensity in each band using ImageJ version 1.42q. Measured transcript levels were normalized to U6 snRNA expression. Samples were run in triplicate. Data are shown as mean ± S.E. (error bars). C, Northern blot analysis of miR-93 expression in high glucose (HG)-treated podocytes compared with normal glucose (NG). D, quantitative analysis of miR-93 expression in podocytes. Measured transcript levels were normalized to U6 snRNA expression. Samples were run in triplicate. Data are shown as mean ± S.E. (error bars). E, reverse transcription-qPCR analysis of miR-93 in podocytes and kidney microvascular endothelial cells (EC) after exposure to high glucose for 24 h. Measured transcript levels were normalized to U6 snRNA expression. Data represent three independent experiments with three replicates each and are shown as mean ± S.E. (error bars).
FIGURE 5.
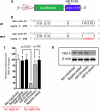
miR-93 targets vegfa. A, schematic diagram of vegfa 3′-UTR reporter construct. B, sequence alignment between miR-93 and mouse vegfa 3′-UTR wild type (wt) and miR-93 mutant (mut). Red color indicates the sequence of the mutated miR-93 binding site. C, HeLa cells transfected with either luc-_vegfa_-wt or luc-_vegfa_-mutant, along with miR-93 mimics (30 n
m
). A nonrelated miRNA (miR-690) was used as control. Luciferase activities were normalized to β-galactosidase activities. Results were obtained from three independent experiments. Data are shown as mean ± S.E. (error bars). NS, nonsignificant. D, Western blot analysis of VEGF-A protein levels in cells transfected with miR-93 mimics.
FIGURE 6.
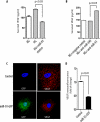
miR-93 represses VEGF-A production in podocytes. A, VEGF released into the culture medium was measured by ELISA after 24 h of exposure to high glucose (HG). Similar experiments were carried out in cells cultured in normal glucose (NG) medium. B, effect of miR-93 inhibitor on VEGF after 24 h of exposure to high glucose was measured by ELISA. C, podocytes were transfected with pEGP-miR-93 plasmid (green), and expression of VEGF (red) was assessed by deconvolution microscopy. Original magnification, ×400. D, quantitative analysis was based on fluorescence intensity of VEGF.
FIGURE 7.
miR-93 inhibits high glucose-induced downstream target genes of VEGF. A, podocytes were transfected with miR-93 mimics with or without VEGF cDNA lacking 3′-UTR. Cells were serum-starved and exposed to high glucose (HG) for 24 h, and α3 collagen (IV) (COL4A3) or fibronectin (FN1). B, mRNAs were assessed by reverse transcription-qPCR. Expression levels of mRNAs were normalized as described under “Experimental Procedures.” Data are shown as mean ± S.E. (error bars).
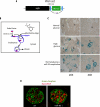
FIGURE 8.
miR-93 regulates VEGF expression in kidneys. A, schematic diagram of the VEGF-LacZ targeting construct. B, mechanism of Endo-Porter system delivery through endocytosis. C, light microscopic images of whole mount X-gal staining demonstrating β-galactosidase activity (blue) in the glomeruli and tubular cells in the kidney sections from adult VEGF-LacZ mice. Kidney cortex pieces were cultured ex vivo in normal glucose, high glucose, or normal glucose in the presence of miR-93 morpholino oligomers. Original magnifications are ×200 and ×400. G, glomerulus. D, immunofluorescence staining of glomerular VEGF with anti-VEGF-A (red) and anti-nephrin (green) antibodies using confocal laser scanning microscopy. Original magnification, ×600.
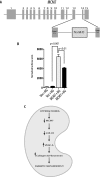
FIGURE 9.
High glucose down-regulates MCM7 promoter activity. A, diagram of the genomic organization of the mouse MCM7 gene. miR-93 is localized within the intron 13 of MCM7. B, luciferase activity of the cloned mouse MCM7 promoter in podocytes. Podocytes were transfected with empty vector (Vec) or mouse MCM7 promoter constructs. Luciferase activity in normal glucose (NG) or high glucose (HG) medium was measured and normalized to β-galactosidase internal control. Quantitative analysis of three independent experiments is shown as mean ± S.E. (error bars). C, proposed mechanism for the putative effects of high glucose on miR-93-mediated VEGF downstream signaling leading to diabetic nephropathy.
Similar articles
- MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy.
Long J, Wang Y, Wang W, Chang BH, Danesh FR. Long J, et al. J Biol Chem. 2011 Apr 1;286(13):11837-48. doi: 10.1074/jbc.M110.194969. Epub 2011 Feb 10. J Biol Chem. 2011. PMID: 21310958 Free PMC article. - microRNA-15b-5p shuttled by mesenchymal stem cell-derived extracellular vesicles protects podocytes from diabetic nephropathy via downregulation of VEGF/PDK4 axis.
Zhao T, Jin Q, Kong L, Zhang D, Teng Y, Lin L, Yao X, Jin Y, Li M. Zhao T, et al. J Bioenerg Biomembr. 2022 Feb;54(1):17-30. doi: 10.1007/s10863-021-09919-y. Epub 2021 Nov 22. J Bioenerg Biomembr. 2022. PMID: 34806156 - MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy.
McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. McArthur K, et al. Diabetes. 2011 Apr;60(4):1314-23. doi: 10.2337/db10-1557. Epub 2011 Feb 28. Diabetes. 2011. PMID: 21357793 Free PMC article. - Mechanistic Pathogenesis of Endothelial Dysfunction in Diabetic Nephropathy and Retinopathy.
Yang J, Liu Z. Yang J, et al. Front Endocrinol (Lausanne). 2022 May 25;13:816400. doi: 10.3389/fendo.2022.816400. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35692405 Free PMC article. Review. - Non-coding RNA regulatory networks in post-transcriptional regulation of VEGFA in cancer.
Fontemaggi G. Fontemaggi G. IUBMB Life. 2023 Jan;75(1):30-39. doi: 10.1002/iub.2620. Epub 2022 May 10. IUBMB Life. 2023. PMID: 35467790 Free PMC article. Review.
Cited by
- Molecular signaling cascade of miRNAs in causing Diabetes Nephropathy.
Padmashree DG, Swamy NR. Padmashree DG, et al. Bioinformation. 2013 Apr 30;9(8):401-8. doi: 10.6026/97320630009401. Print 2013. Bioinformation. 2013. PMID: 23750089 Free PMC article. - The regulation and function of microRNAs in kidney diseases.
Wei Q, Mi QS, Dong Z. Wei Q, et al. IUBMB Life. 2013 Jul;65(7):602-14. doi: 10.1002/iub.1174. IUBMB Life. 2013. PMID: 23794512 Free PMC article. Review. - MicroRNAs and the glomerulus.
Kato M, Park JT, Natarajan R. Kato M, et al. Exp Cell Res. 2012 May 15;318(9):993-1000. doi: 10.1016/j.yexcr.2012.02.034. Epub 2012 Mar 5. Exp Cell Res. 2012. PMID: 22421514 Free PMC article. Review. - The Role of MicroRNAs in the Pathogenesis of Diabetic Nephropathy.
Tang J, Yao D, Yan H, Chen X, Wang L, Zhan H. Tang J, et al. Int J Endocrinol. 2019 Dec 1;2019:8719060. doi: 10.1155/2019/8719060. eCollection 2019. Int J Endocrinol. 2019. PMID: 31885563 Free PMC article. Review. - MicroRNA-21 orchestrates high glucose-induced signals to TOR complex 1, resulting in renal cell pathology in diabetes.
Dey N, Das F, Mariappan MM, Mandal CC, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. Dey N, et al. J Biol Chem. 2011 Jul 22;286(29):25586-603. doi: 10.1074/jbc.M110.208066. Epub 2011 May 25. J Biol Chem. 2011. PMID: 21613227 Free PMC article.
References
- Eremina V., Baelde H., Quaggin S. (2007) Nephron Physiol. 106, 32–37 - PubMed
- Chen S., Ziyadeh F. N. (2008) Curr. Diab. Rep. 8, 470–476 - PubMed
- Sugimoto H., Hamano Y., Charytan D., Cosgrove D., Kieran M., Sudhakar A., Kalluri R. (2003) J. Biol. Chem. 278, 12605–12608 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK067604/DK/NIDDK NIH HHS/United States
- R01 DK078900/DK/NIDDK NIH HHS/United States
- R01DK067604/DK/NIDDK NIH HHS/United States
- R01 DK091310/DK/NIDDK NIH HHS/United States
- R01DK078900/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous