Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss - PubMed (original) (raw)
Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss
Jun Yang et al. PLoS Genet. 2010.
Abstract
Mutations in whirlin cause either Usher syndrome type II (USH2), a deafness-blindness disorder, or nonsyndromic deafness. The molecular basis for the variable disease expression is unknown. We show here that only the whirlin long isoform, distinct from a short isoform by virtue of having two N-terminal PDZ domains, is expressed in the retina. Both long and short isoforms are expressed in the inner ear. The N-terminal PDZ domains of the long whirlin isoform mediates the formation of a multi-protein complex that includes usherin and VLGR1, both of which are also implicated in USH2. We localized this USH2 protein complex to the periciliary membrane complex (PMC) in mouse photoreceptors that appears analogous to the frog periciliary ridge complex. The latter is proposed to play a role in photoreceptor protein trafficking through the connecting cilium. Mice carrying a targeted disruption near the N-terminus of whirlin manifest retinal and inner ear defects, reproducing the clinical features of human USH2 disease. This is in contrast to mice with mutations affecting the C-terminal portion of whirlin in which the phenotype is restricted to the inner ear. In mice lacking any one of the USH2 proteins, the normal localization of all USH2 proteins is disrupted, and there is evidence of protein destabilization. Taken together, our findings provide new insights into the pathogenic mechanism of Usher syndrome. First, the three USH2 proteins exist as an obligatory functional complex in vivo, and loss of one USH2 protein is functionally close to loss of all three. Second, defects in the three USH2 proteins share a common pathogenic process, i.e., disruption of the PMC. Third, whirlin mutations that ablate the N-terminal PDZ domains lead to Usher syndrome, but non-syndromic hearing loss will result if they are spared.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
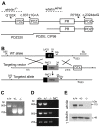
Figure 1. Whirlin knockout mice were generated.
(A) A schematic diagram illustrating the long and short isoforms of whirlin. The dashed lines indicate the deletion regions of the whirlin gene in whirlin knockout (whirlin−/−) and whirler (whirlinwi_/wi_) mice. The asterisks indicate the mutations of the whirlin gene in humans. The bottom solid lines indicate the antigen regions of various whirlin antibodies. PDZ, postsynaptic density 95/discs large/zonula occludens 1; PR, proline-rich region. (B) Targeting strategy for disrupting the whirlin gene. PCR primers for identification of the mutant and wild-type alleles are shown as arrowheads. E1, exon 1; neo, neomycin, the positive selective marker; DTA, diphtheria toxin expression cassette, the negative selective marker. (C) Identification of the mutant allele by genomic PCR using primers G1, G5r and 3A. (D) RT-PCR analysis shows loss of the first exon of whirlin transcripts in the homozygous mutant retina. Whirlin mRNA transcripts were reverse transcribed and amplified using primers located on exon 1 and 6 (top panel), exon 2 and 6 (middle panel), and exon 11 and 12 (bottom panel). Exon 12 is the last exon of the whirlin gene. NC, negative controls with water instead of DNA samples. (E) The whirlin long isoform was completely knocked out in the retina of homozygous mutants as shown by immunoblotting. γ–tubulin served as a loading control. +/+, wild-types; −/−, whirlin knockout homozygotes; +/−, whirlin knockout heterozygotes.
Figure 2. Whirlin expresses its long isoform in the retina.
(A) Western blotting analysis of retinal lysates. All CIP98, RbPDZIE, and ChPDZIE antibodies detected only the whirlin long isoform. The lower bands on the blots of RbPDZIE and ChPDZIE are nonspecific, because the bands on these two blots have different molecular weights and they are present in the retina of whirlin knockout and whirler mice. (B) Western blotting of immunoprecipitates from the retina (left) and the inner ear (right). The ChPRZIE antibody detected only the whirlin long isoform in the RbPDZIE immunoprecipitate from the retina, but detected both the long and short isoforms from the inner ear. The ChPDZ320 antibody detected only the whirlin long isoform but not an N-terminal whirlin fragment in the RbPDZ320 immunoprecipitate from the retina. RbPDZIE and ChPDZIE, the rabbit and chicken PDZIE (Figure 1A) antibody, respectively; RbPDZ320 and ChPDZ320, the rabbit and chicken PDZ320 (Figure 1A) antibody, respectively; RbIgG, rabbit immunoglobulin, a negative control. WT, wild-types; whirlin−/−, whirlin knockout mutants; whirlin_wi/wi_, whirler mutants.
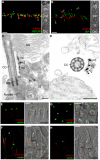
Figure 3. Whirlin is localized at the PMC and at the PRC.
Whirlin is localized at the PMC in mouse photoreceptors (A–D) and at the PRC in frog photoreceptors (E–H). (A) Whirlin (red, arrowheads) was localized closely next to the connecting cilia, marked by RPGR (green), between the inner and outer segments in the mouse retina. The corresponding phase contrast image is attached on the right. (B) Whirlin (red, arrowheads) was localized obliquely below the signals of RP1 (green) in mouse photoreceptors. The corresponding phase contrast image is attached on the right. (C,D) Gold labels of whirlin (arrowheads) are present on the plasma membrane of inner segments facing the connecting cilia as shown by the longitudinal (C) and transverse (D) views of immunoelectron microscopy. (E–H) Whirlin (green) was localized above the basal bodies marked by γ-tubulin (red, E,H) and below the axonemal microtubules marked by acetylated α-tubulin (red, F) in the longitudinal views of frog photoreceptors. It appears as circles surrounding the basal bodies in the transverse view (G). The distribution of whirlin is same in both rod and cone photoreceptors (F,H). The merged images on the right (E–H) are superimposed immunofluorescent images and their corresponding phase contrast images. OS, outer segments; CC, connecting cilia; IS, inner segments; DIC, differential interference contrast image; BB, basal body. Scale bars, 5 µm (A,B,E–H), 200 nm (C,D).
Figure 4. Whirlin directly interacts with usherin.
(A) A yeast two-hybrid analysis demonstrates that mouse whirlin, through its N-terminal two PDZ domains (whirlin N-term), interacts with the C-terminal fragment of mouse usherin. The “+” sign denotes growth of large, white yeast colonies and “−” denotes absence of colonies. This experiment was conducted in two orientations. (B) A GST pull-down assay demonstrates that the intact C-terminal fragments of mouse and frog usherin, but not the mutant ones, which lack the PDZ-binding motif, were able to pull down the endogenous whirlin from the retinal lysate, indicating that the PDZ-binding motif of usherin is involved in the interaction between whirlin and usherin. The arrows on the GST blot indicate the positions of mouse and frog GST-fused usherin fragments and GST. The multiple bands below the mouse and frog GST-fused usherin fragments are their degraded products.
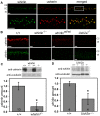
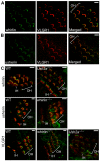
Figure 5. Whirlin and usherin are colocalized in photoreceptors.
(A) Whirlin (green) and usherin (red) were colocalized at the PMC in mouse photoreceptors. The bottom panels are the enlarged view of the region marked by the frame in the merged image on the top. (B) The signals of usherin (red, top) and whirlin (green, bottom) were mislocalized from the PMC in whirlin knockout (whirlin−/−), whirler mutant (whirlin_wi/wi_) and Ush2a knockout (Ush2a−/−) mice (see results section for details). OS, outer segments; CC, connecting cilia; IS, inner segments. Scale bars (A,B), 5 µm. (C) The amount of usherin was reduced by about 80% in the retina of whirlin knockout mice as analyzed by immunoblotting. (D) The amount of whirlin was reduced by about 50% in the retina of Ush2a knockout mice as analyzed by immunoblotting. The images on the top of the bar charts in (C,D) are the representative western blots of usherin and whirlin, respectively. The signals of α-tubulin were used as a loading control. Error bars in (C,D) represent the standard error of the mean. The numbers in the bottom of each bar (C,D) are the numbers of animals analyzed.
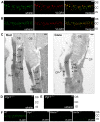
Figure 6. VLGR1 is colocalized with the complex of whirlin and usherin at the PMC in photoreceptors.
VLGR1 (red) was colocalized with whirlin (green, A) and usherin (green, B) at the PMC in mouse photoreceptors. Immunoelectron microscopy (C) demonstrated that the gold labels of VLGR1 were present at the plasma membrane of the apical inner segment facing the connecting cilium, the PMC, in both mouse rod and cone photoreceptors (arrowheads). The signals of whirlin (D) and usherin (E) disappeared at the PMC in Vlgr1 knockout photoreceptors. The signals of VLGR1 diminished in whirlin and Ush2a knockout mice (F). The staining of VLGR1 in wild-type (WT) and Vlgr1 knockout mice serves as a positive and negative control, respectively. OS, outer segments; CC, connecting cilia; BB, basal bodies; Rt, the rootlet; IS, inner segments; CP, calycal processes. Scale bars, 5 µm (A,B,D–F) and 200 nm (C).
Figure 7. Whirlin, usherin and VLGR1 are colocalized in stereocilia of inner ear hair cells at postnatal day 4.
Whirlin (green, A) and usherin (green, B) were colocalized with VLGR1 (red) in stereocilia in the mouse cochlea. Signals of whirlin (green, top row in C) and usherin (green, middle row in C) diminished in hair cell stereocilia (phalloidin, red) in the Ush2a and whirlin knockout cochleas, respectively. Signals of VLGR1 (green, bottom row in C) diminished at the hair cell stereocilia in both whirlin and Ush2a knockout cochleas. OH, outer hair cells; IH, inner hair cells. Scale bars, 5 µm.
Figure 8. Whirlin knockout mice have morphological defects around the PMC in photoreceptors revealed by electron microscopy.
(A) A representative image showing the normal ultrastructure around the PMC in the wild-type photoreceptor (whirlin+/+). (B–F) In whirlin knockout mice (whirlin−/−), abnormal distance (E) and membrane fusion (empty arrows, B–E) between the apical inner segment and the connecting cilium were found. In addition, a large amount of vacuoles (filled arrows, D–F) were accumulated around the PMC. (D,D′) show the same cell at different sectioning levels. OS, outer segments; CC, connecting cilia; IS, inner segments. Scale bars, 200 nm. (G) All the above abnormalities exist in a small fraction of photoreceptors randomly distributed in the retina of whirlin knockout mice. The number of mice analyzed in each group is indicated in the bottom of each bar. Mean ± SEM; **, p<0.01.
Figure 9. Retinal degeneration becomes apparent in whirlin knockout mice at 28–33 months of age.
(A) Representative dark-adapted ERG tracings show reduced a- and b-waves in response to a series of white light stimuli in whirlin knockout mice at this age. (B) Representative 1-µm retinal sections stained with toluidine blue show shortened OS and reduced ONL in whirlin knockout mice. Scale bars, 5 µm. (C) Representative 1-µm retinal sections stained with toluidine blue show comparable thickness of OS and ONL between whirler mice and age-matched WT (whirlin+/+) mice. Scale bars, 5 µm. (D) Measurement of OS length and ONL thickness at different locations along the vertical meridian in whirlin knockout retinas. The number of mice measured at each location in each group is indicated above or below the lines. The error bar represents the standard error of the mean. *, p<0.05. Whirlin heterozygous littermates were used as a control in these studies. (E) Measurement of OS length and ONL thickness at different locations along the vertical meridian in whirler retinas. The numbers of mice measured at each point in whirler and age-matched wild-type cohorts are indicated below and above the lines, respectively. The error bar represents the standard error of the mean. RPE, retinal pigment epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer.
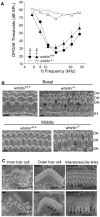
Figure 10. Whirlin knockout mice have non-progressive hearing defects.
(A) DPOAE analysis demonstrates that whirlin knockout mice have profound hearing loss at all stimulus frequencies, as measured at either 2 or 9 months of age. (B) Scanning electron microscopy shows scattered loss of outer hair cells (arrows) in the basal turn and dysmorphology of the stereocilia bundles in all cochlear regions (e.g. open arrowheads) in whirlin knockout mice. (C) At high magnification, in whirlin knockout mice, the inner hair cell stereocilia appear normal (left column); the outer hair cells show patchy loss of stereocilia in the innermost (shortest) row of the hair bundle (arrowheads, middle column); the interstereocilia links of the outer hair cells appear normal (arrowheads, right column). OH, outer hair cells; IH, inner hair cells. Scale bars, 5 µm (B) and 1 µm (C).
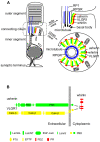
Figure 11. Schematic diagrams illustrate the USH2 multi-protein complex at the PMC in photoreceptors.
(A) Localization of the complex of whirlin (red), usherin (green) and VLGR1 (yellow) at the PMC in mammalian photoreceptors. On the left is a whole-cell view. On the right are the enlarged longitudinal and cross-sectional views. (B) The interactions among whirlin, usherin and VLGR1. PR, proline-rich region; LamGL, LamG-like jellyroll fold domain; LamNT, laminin N-terminal domain; EGF-Lam, laminin-type epidermal growth factor-like domain; FN3, fibronectin type 3 domain; LamG, laminin G domain; PBD, PDZ-binding motif; Calx-β, domains in Na-Ca exchangers and integrin-beta4; PTX, pentraxin domain; EPTP, epitempin domain.
Similar articles
- Whirlin replacement restores the formation of the USH2 protein complex in whirlin knockout photoreceptors.
Zou J, Luo L, Shen Z, Chiodo VA, Ambati BK, Hauswirth WW, Yang J. Zou J, et al. Invest Ophthalmol Vis Sci. 2011 Apr 12;52(5):2343-51. doi: 10.1167/iovs.10-6141. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21212183 Free PMC article. - A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss.
Ebermann I, Scholl HP, Charbel Issa P, Becirovic E, Lamprecht J, Jurklies B, Millán JM, Aller E, Mitter D, Bolz H. Ebermann I, et al. Hum Genet. 2007 Apr;121(2):203-11. doi: 10.1007/s00439-006-0304-0. Epub 2006 Dec 15. Hum Genet. 2007. PMID: 17171570 - Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II.
Wang L, Zou J, Shen Z, Song E, Yang J. Wang L, et al. Hum Mol Genet. 2012 Feb 1;21(3):692-710. doi: 10.1093/hmg/ddr503. Epub 2011 Nov 2. Hum Mol Genet. 2012. PMID: 22048959 Free PMC article. - Usher syndrome and non-syndromic deafness: Functions of different whirlin isoforms in the cochlea, vestibular organs, and retina.
Mathur PD, Yang J. Mathur PD, et al. Hear Res. 2019 Apr;375:14-24. doi: 10.1016/j.heares.2019.02.007. Epub 2019 Feb 22. Hear Res. 2019. PMID: 30831381 Free PMC article. Review. - Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease.
Reiners J, Nagel-Wolfrum K, Jürgens K, Märker T, Wolfrum U. Reiners J, et al. Exp Eye Res. 2006 Jul;83(1):97-119. doi: 10.1016/j.exer.2005.11.010. Epub 2006 Mar 20. Exp Eye Res. 2006. PMID: 16545802 Review.
Cited by
- Molecular basis for photoreceptor outer segment architecture.
Goldberg AF, Moritz OL, Williams DS. Goldberg AF, et al. Prog Retin Eye Res. 2016 Nov;55:52-81. doi: 10.1016/j.preteyeres.2016.05.003. Epub 2016 Jun 1. Prog Retin Eye Res. 2016. PMID: 27260426 Free PMC article. Review. - A large-scale screening identified in USH2A gene the P3272L founder pathogenic variant explaining familial Usher syndrome in Sardinia, Italy.
Serra R, Rallo V, Steri M, Olla S, Piras MG, Marongiu M, Gorospe M, Schlessinger D, Pinna A, Fiorillo E, Cucca F, Angius A. Serra R, et al. BMC Ophthalmol. 2024 Jul 23;24(1):306. doi: 10.1186/s12886-024-03578-4. BMC Ophthalmol. 2024. PMID: 39044131 Free PMC article. - Comparison of structural progression between ciliopathy and non-ciliopathy associated with autosomal recessive retinitis pigmentosa.
Takahashi VKL, Xu CL, Takiuti JT, Apatoff MBL, Duong JK, Mahajan VB, Tsang SH. Takahashi VKL, et al. Orphanet J Rare Dis. 2019 Aug 1;14(1):187. doi: 10.1186/s13023-019-1163-9. Orphanet J Rare Dis. 2019. PMID: 31370859 Free PMC article. - Adenylyl cyclase 6 plays a minor role in the mouse inner ear and retina.
Mathur PD, Zou J, Neiswanger G, Zhu D, Wang Y, Almishaal AA, Vashist D, Hammond HK, Park AH, Yang J. Mathur PD, et al. Sci Rep. 2023 May 1;13(1):7075. doi: 10.1038/s41598-023-34361-y. Sci Rep. 2023. PMID: 37127773 Free PMC article. - Successful large gene augmentation of USH2A with non-viral episomal vectors.
Toms M, Toualbi L, Almeida PV, Harbottle R, Moosajee M. Toms M, et al. Mol Ther. 2023 Sep 6;31(9):2755-2766. doi: 10.1016/j.ymthe.2023.06.012. Epub 2023 Jun 19. Mol Ther. 2023. PMID: 37337429 Free PMC article.
References
- Beaty TH, Boughman JA. Problems in detecting etiological heterogeneity in genetic disease illustrated with retinitis pigmentosa. Am J Med Genet. 1986;24:493–504. - PubMed
- Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36:595–603. - PubMed
- Adato A, Vreugde S, Joensuu T, Avidan N, Hamalainen R, et al. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur J Hum Genet. 2002;10:339–350. - PubMed
- Geller SF, Guerin KI, Visel M, Pham A, Lee ES, et al. CLRN1 is nonessential in the mouse retina but is required for cochlear hair cell development. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000607. - DOI - PMC - PubMed
- Petit C. Usher syndrome: from genetics to pathogenesis. Annu Rev Genomics Hum Genet. 2001;2:271–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY10309/EY/NEI NIH HHS/United States
- R01 DC00188/DC/NIDCD NIH HHS/United States
- P30 DC005209/DC/NIDCD NIH HHS/United States
- P30 EY014104/EY/NEI NIH HHS/United States
- R01 EY010309/EY/NEI NIH HHS/United States
- R01 DC000188/DC/NIDCD NIH HHS/United States
- P30 DC05209/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases