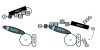Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets - PubMed (original) (raw)
Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets
Christopher Hooper et al. BMC Biol. 2010.
Abstract
Background: Spartin protein is involved in degradation of epidermal growth factor receptor and turnover of lipid droplets and a lack of expression of this protein is responsible for hereditary spastic paraplegia type 20 (SPG20). Spartin is a multifunctional protein that associates with many cellular organelles, including lipid droplets. Recent studies showed that spartin interacts with E3 ubiquitin ligases that belong to the neural precursor cell-expressed developmentally downregulated gene (Nedd4) family, including atrophin-1-interacting protein 4 (AIP4/ITCH). However, the biological importance of the spartin-AIP4 interaction remains unknown.
Results: In this study, we show that spartin is not a substrate for AIP4 activity and that spartin's binding to AIP4 significantly increases self-ubiquitination of this E3 ligase, indicating that spartin disrupts the AIP4 autoinhibitory intramolecular interaction. Correspondingly, spartin has a seven times higher binding affinity to the WW region of AIP4 than the binding of the WW region has to the catalytic homologues of the E6-associated protein C-terminus (HECT) domain, as measured by enzyme-linked immunosorbent assay. We also show that spartin recruits AIP4 to lipid droplets and promotes ubiquitination of lipid droplet-associated protein, adipophilin, which regulates turnover of lipid droplets.
Conclusions: Our findings demonstrate that spartin acts as an adaptor protein that activates and recruits AIP4 E3 ubiquitin ligase to lipid droplets and by this means regulates the level of ubiquitination of adipophilin and potentially other lipid-associated proteins. We propose that this is one of the mechanisms by which spartin regulates lipid droplet turnover and might contribute to the pathology of SPG20.
Figures
Figure 1
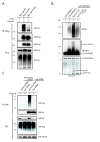
Atrophin-1-interacting protein 4 (AIP4) does not ubiquitinate spartin. (a) HeLa cells were transfected with Myc-wild-type spartin together with an empty vector (lane 1), hemagglutinin (HA)-ubiquitin (lane 2), HA-ubiquitin and Flag-AIP4 (lane 3), or HA-ubiquitin and Flag-AIP4C830A (lane 4). TCL = total cell lysates. WB = western blot. An asterisk (*) indicates crossreactive proteins. Sizes of protein standards are indicated to the left in kDa. (b) Immunoprecipitates of HA-wild-type, mutant PY/AA, or 211-666 fragments of spartin and Flag-ubiquitin analyzed by immunoblotting with anti-Flag (upper panel) or anti-HA (lower panel) antibodies. (c) HeLa cells were transfected with Flag-AIP4 alone or together with HA-wild-type spartin, HA-spartin PY/AA, or HA-spartin 211-666 fragment. Cell lysates were immunoprecipitated (IP) with anti-HA antibodies and immunoblotted with anti-Flag (upper panel) or anti-HA (lower panel) antibodies. An asterisk (*) and kDa are indicated as in A. (d) Schematic diagram of spartin constructs and their status of ubiquitination and binding to AIP4.
Figure 2
Spartin recruits atrophin-1-interacting protein 4 (AIP4) to lipid droplets. (a) HeLa cells were transfected with hemagglutinin (HA)-spartin and immunostained for HA in blue (A1) and stained for lipid droplets with Bodipy 493/503 in green (A3). Merged image is shown in A4. The boxed areas are enlarged in the insets. (b) HeLa cells were transfected with Flag-AIP4 and immunostained for Flag in red (B2) and stained for lipid droplets with Bodipy 493/503 in green (B3). Merged image is shown in B4. The boxed areas are enlarged in the insets. (c) HeLa cells were cotransfected with HA-spartin and Flag-AIP4 and immunostained for HA in blue (C1), for Flag in red (C2), and for lipid droplets with Bodipy 493/503 in green (C3). Merged image is shown in C4. The boxed areas are enlarged in the insets. (d) HeLa cells were cotransfected with HA-spartin PY/AA and Flag-AIP4 and immunostained for HA in blue (D1), for Flag in red (D2), and for lipid droplets with Bodipy 493/503 in green (D3). Merged image is shown in D4. The boxed areas are enlarged in the insets. Bars = 20 μm. (e) Colocalization of indicated expressed proteins with lipid droplets was scored for 30 cells in each of 3 independent experiments. Transfected vectors are indicated with '+' below each column.
Figure 3
Spartin increases activity of atrophin-1-interacting protein 4 (AIP4) and promotes ubiquitination of adipophilin. (a) HeLa cells were cotransfected with Myc-wild-type spartin together with hemagglutinin (HA)-ubiquitin and an empty vector (lane 1), or Flag-AIP4 (lane 2), or Myc-spartin PY/AA with HA-ubiquitin and Flag-AIP4 (lane 3). Cell lysates were boiled in sodium dodecyl sulfate (SDS) before immunoprecipitation (IP) with anti-Flag antibodies. Levels of HA-ubiquitin and Flag-AIP4 in immunoprecipitates and total cell lysates (TCL) were analyzed by immunoblotting. (b) Levels of in vitro ubiquitination assay of glutathione S-transferase (GST)-AIP4ΔC2 alone (lane 2), with spartin155-367 (lane 3), or mutant spartin 155-367PY/AA (lane 4) determined by immunoblot with anti-ubiquitin antibodies (upper panel). All reactions (except lane 1) were carried out in the presence of E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, ATP, and ubiquitin. Coomassie blue stained gel (bottom panel). (c) In vivo ubiquitination assay of HA-adipophilin, also known as adipose differentiation-related protein (ADRP) in HeLa cells transfected with either HA-empty vector or HA-ADRP, and control or spartin small interfering RNA (siRNA) and Flag-ubiquitin and treated with 300 μM of oleic acid. Cell lysates were immunoprecipitated with anti-HA antibodies and immunoblotted with anti-Flag or anti-HA antibodies.
Figure 4
Binding of proline-rich region (PRR)/WWI-IV to the homologues of the E6-associated protein C-terminus (HECT) domain of atrophin-1-interacting protein 4 (AIP4) and wild-type (WT) spartin. (a) Binding isotherm of the glutathione S-transferase (GST)-HECT protein to PRR/WWI-IV. AU = arbitrary units. (b) Binding isotherm of the GST-WT spartin 155-367 protein to PRR/WWI-IV. (c) Inhibition of GST-HECT binding to PRR/WWI-IV by WT spartin. All experiments were repeated three times. Data are shown as mean ± SD with an R2 ≥ 0.95.
Figure 5
Model of spartin's role in the activation and recruitment of atrophin-1-interacting protein 4 (AIP4) to lipid droplets and subsequent adipophilin ubiquitination. AIP4 in the absence of binding to spartin is in an autoinhibited conformation (left panel). Our heuristic model predicts that spartin, via its PPAY motif, recruits AIP4 to lipid droplets and alters AIP4 conformation, which leads to its increased enzymatic activity. The ubiquitination of resident proteins present on lipid droplets, such as adipophilin, is facilitated by the spartin-mediated recruitment and activation of AIP4 (right panel). I, II, III, IV = WW-I, WW-II, WW-III, and WW-IV domains; ADRP = adipophilin (also known as adipose differentiation-related protein); C2 = calcium binding domain; HECT = homologues of the E6-associated protein C-terminal domain; LD = lipid droplet; PPAY = PPAY motif of spartin; PRR = proline-rich domain; Ub = ubiquitin.
Comment in
- Regulation of lipid droplet turnover by ubiquitin ligases.
Alberts P, Rotin D. Alberts P, et al. BMC Biol. 2010 Jul 19;8:94. doi: 10.1186/1741-7007-8-94. BMC Biol. 2010. PMID: 20646264 Free PMC article.
Similar articles
- Endogenous spartin (SPG20) is recruited to endosomes and lipid droplets and interacts with the ubiquitin E3 ligases AIP4 and AIP5.
Edwards TL, Clowes VE, Tsang HT, Connell JW, Sanderson CM, Luzio JP, Reid E. Edwards TL, et al. Biochem J. 2009 Sep 14;423(1):31-9. doi: 10.1042/BJ20082398. Biochem J. 2009. PMID: 19580544 Free PMC article. - Regulation of lipid droplet turnover by ubiquitin ligases.
Alberts P, Rotin D. Alberts P, et al. BMC Biol. 2010 Jul 19;8:94. doi: 10.1186/1741-7007-8-94. BMC Biol. 2010. PMID: 20646264 Free PMC article. - A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover.
Eastman SW, Yassaee M, Bieniasz PD. Eastman SW, et al. J Cell Biol. 2009 Mar 23;184(6):881-94. doi: 10.1083/jcb.200808041. J Cell Biol. 2009. PMID: 19307600 Free PMC article. - ITCH as a potential therapeutic target in human cancers.
Yin Q, Wyatt CJ, Han T, Smalley KSM, Wan L. Yin Q, et al. Semin Cancer Biol. 2020 Dec;67(Pt 2):117-130. doi: 10.1016/j.semcancer.2020.03.003. Epub 2020 Mar 10. Semin Cancer Biol. 2020. PMID: 32165318 Free PMC article. Review. - Spartin: At the crossroad between ubiquitination and metabolism in cancer.
Cusenza VY, Bonora E, Amodio N, Frazzi R. Cusenza VY, et al. Biochim Biophys Acta Rev Cancer. 2022 Nov;1877(6):188813. doi: 10.1016/j.bbcan.2022.188813. Epub 2022 Oct 1. Biochim Biophys Acta Rev Cancer. 2022. PMID: 36195276 Review.
Cited by
- Mitochondria: the next (neurode)generation.
Schon EA, Przedborski S. Schon EA, et al. Neuron. 2011 Jun 23;70(6):1033-53. doi: 10.1016/j.neuron.2011.06.003. Neuron. 2011. PMID: 21689593 Free PMC article. Review. - Detection of SPG20 gene promoter-methylated DNA, as a novel epigenetic biomarker, in plasma for colorectal cancer diagnosis using the MethyLight method.
Rezvani N, Alibakhshi R, Vaisi-Raygani A, Bashiri H, Saidijam M. Rezvani N, et al. Oncol Lett. 2017 May;13(5):3277-3284. doi: 10.3892/ol.2017.5815. Epub 2017 Mar 7. Oncol Lett. 2017. PMID: 28521434 Free PMC article. - Mitochondrial energy metabolism is required for lifespan extension by the spastic paraplegia-associated protein spartin.
Ring J, Rockenfeller P, Abraham C, Tadic J, Poglitsch M, Schimmel K, Westermayer J, Schauer S, Achleitner B, Schimpel C, Moitzi B, Rechberger GN, Sigrist SJ, Carmona-Gutierrez D, Kroemer G, Büttner S, Eisenberg T, Madeo F. Ring J, et al. Microb Cell. 2017 Nov 30;4(12):411-422. doi: 10.15698/mic2017.12.603. Microb Cell. 2017. PMID: 29234670 Free PMC article. - Spg20-/- mice reveal multimodal functions for Troyer syndrome protein spartin in lipid droplet maintenance, cytokinesis and BMP signaling.
Renvoisé B, Stadler J, Singh R, Bakowska JC, Blackstone C. Renvoisé B, et al. Hum Mol Genet. 2012 Aug 15;21(16):3604-18. doi: 10.1093/hmg/dds191. Epub 2012 May 22. Hum Mol Genet. 2012. PMID: 22619377 Free PMC article. - Lipid droplets and cellular lipid metabolism.
Walther TC, Farese RV Jr. Walther TC, et al. Annu Rev Biochem. 2012;81:687-714. doi: 10.1146/annurev-biochem-061009-102430. Epub 2012 Apr 13. Annu Rev Biochem. 2012. PMID: 22524315 Free PMC article. Review.
References
- Patel H, Cross H, Proukakis C, Hershberger R, Bork P, Ciccarelli FD, Patton MA, McKusick VA, Crosby AH. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat Genet. 2002;31:347–348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials