The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3 - PubMed (original) (raw)
. 2010 Jun 15;24(12):1253-65.
doi: 10.1101/gad.566910. Epub 2010 May 26.
Affiliations
- PMID: 20504901
- PMCID: PMC2885661
- DOI: 10.1101/gad.566910
The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3
Pascal Drané et al. Genes Dev. 2010.
Abstract
The histone variant H3.3 marks active chromatin by replacing the conventional histone H3.1. In this study, we investigate the detailed mechanism of H3.3 replication-independent deposition. We found that the death domain-associated protein DAXX and the chromatin remodeling factor ATRX (alpha-thalassemia/mental retardation syndrome protein) are specifically associated with the H3.3 deposition machinery. Bacterially expressed DAXX has a marked binding preference for H3.3 and assists the deposition of (H3.3-H4)(2) tetramers on naked DNA, thus showing that DAXX is a H3.3 histone chaperone. In DAXX-depleted cells, a fraction of H3.3 was found associated with the replication-dependent machinery of deposition, suggesting that cells adapt to the depletion. The reintroduced DAXX in these cells colocalizes with H3.3 into the promyelocytic leukemia protein (PML) bodies. Moreover, DAXX associates with pericentric DNA repeats, and modulates the transcription from these repeats through assembly of H3.3 nucleosomes. These findings establish a new link between the PML bodies and the regulation of pericentric DNA repeat chromatin structure. Taken together, our data demonstrate that DAXX functions as a bona fide histone chaperone involved in the replication-independent deposition of H3.3.
Figures
Figure 1.
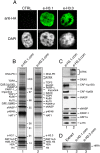
Immunopurification of e-H3.1 and e-H3.3 deposition complexes from soluble nuclear fractions. (A) Stable expression of e-H3.1 and e-H3.3 in HeLa cells. Cells expressing e-H3.1 or e-H3.3 and control cells (CTRL) were stained with anti-HA (top) and DAPI (bottom). (B) Silver staining of proteins associated with e-H3.1 (lane 1) and e-H3.3 NCs (lane 2). The complexes containing e-H3.1 (
e-H3.1.com
) and e-H3.3 (
e-H3.3.com
) were purified by double immunoaffinity from soluble nuclear extracts (NCs). Polypeptides identified by mass spectrometry analysis and the positions of molecular size markers are indicated. (C) DAXX and ATRX proteins are specific to the e-H3.3 NC. The e-H3.1 (lane 1) and e-H3.3 (lane 2) complexes were analyzed by immunoblotting with the indicated antibodies. (D) HIRA is not associated with H3.3. The e-H3.1 (lane 2) and e-H3.3 (lane 3) NCs were analyzed by immunoblotting with anti-HIRA antibody. (Lane 1) HeLa whole-cell extract was used as a control.
Figure 2.
DAXX is stably associated with the H3.3, but not with the H3.1 complex. (A) Silver staining of nuclear e-H3.1 complex fractionated on a glycerol gradient. The e-H3.1 NC purified by double affinity was separated on a glycerol gradient. Fractions were pooled as indicated at the top of the gel. The approximate molecular weight of the different subcomplexes was estimated using the NativeMark molecular weight marker (MWM; Invitrogen). (B) Silver staining of nuclear e-H3.3 complex fractionated on a glycerol gradient. Experiments were performed as described in A. (C) Silver staining of pooled fractions containing e-H3.1 and e-H3.3 nuclear subcomplexes (LNC and HNC) and of e-H3.1 and e-H3.3 CCs. (D) Immunoblotting of pooled fractions containing e-H3.1 and e-H3.3 nuclear subcomplexes (LNC and HNC) and of e-H3.1 and e-H3.3 CCs with the indicated antibodies. Input fraction (extract) is shown for the blot with anti-HIRA.
Figure 3.
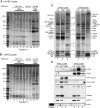
DAXX preferentially associates with H3.3 in vitro and in vivo. (A) Primary structure of DAXX. DAXX contains several putative domains: two paired amphipathic helices (PAH1 and PAH2), a coiled-coil (CC), an acidic domain (acidic), a Ser/Pro/Thr rich domain (S/P/T-rich), and an rtt106-like domain (rtt106). (B) DAXX preferentially associates with H3.3 in vitro. GST-DAXX, immobilized on glutathione-agarose, was incubated with recombinant histones H3.1–H4 (lanes 3_–_5) or H3.3–H4 (lanes 7_–_9). Bead-bound complexes were washed with the indicated concentration of NaCl. Eluted proteins were fractionated on SDS-PAGE and stained with colloidal blue. The input lanes (INP) represent the amount of proteins used for the pull-down. (C) The central part of DAXX contains a high affinity H3.3-interacting domain. N-terminal (1–302), central (302–495), and C-terminal (495–740) regions of DAXX were produced as GST fusion proteins. The fusion proteins (lanes 2_–_7) and GST alone (lane 1), immobilized on glutathione-agarose resin, were incubated with tetramers containing epitope-tagged H3.3. (Top) After washing with either 0.25 or 1 M NaCl, the resin-bound tetramers were analyzed by immunoblotting using anti-HA antibody. (Bottom) To compare the levels of the GST fusions used for the pull-down, the blot was first stained with Ponceau red. The input lane (i) represents 40% of the amount of tetramers used for the pull-down. (D) Silver staining of DAXX and HIRA complexes (
e-DAXX.com
and
e-HIRA.com
) purified by double immunoaffinity from either cytoplasmic extract (CC) or soluble nuclear extract (NC). The polypeptides identified by mass spectrometry analysis are indicated. (E) The DAXX complex, but not the HIRA complex, contains H3.3. The e-DAXX (lanes 2,3) and e-HIRA (lanes 4,5) complexes were analyzed by immunoblotting with anti-HA (top) and anti-H3.3 (bottom) antibodies. (Lane 1) Total histones purified from HeLa cells were used as control.
Figure 4.
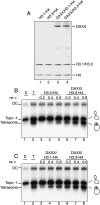
DAXX favors deposition of H3.3 in vitro. (A) Purification and reconstitution of recombinant DAXX/histone complex. Histones H3.1–H4 (lane 1) or H3.3–H4 (lane 2) and full-length DAXX were expressed in bacteria and mixed at equimolar ratio (lanes 3,4). (B) DAXX facilitates the deposition of (H3.3–H4)2 tetramers on DNA. Negatively supercoiled DNA corresponding to topoisomer −1 was incubated with increasing amounts of (H3.3–H4)2 tetramers (at the indicated histone/DNA ratio, rw) either in the presence (lanes 6_–_8) or the absence (lanes 3_–_5) of equimolar (to the tetramers) amounts of GST-DAXX. The reaction products were then analyzed on native 4.5% polyacrylamide gel. (Lane 1) Topoisomer −1 DNA. (Lane 2) (H3.3–H4)2 tetrasomes reconstituted on topoisomer −1 by salt dialysis. Positions of the open circular DNA (OC), the naked topoisomer −1 DNA, and the (H3.3–H4)2 tetrasome are indicated. (C) DAXX deposits more efficiently (H3.3–H4)2 than (H3.1–H4)2 tetramers. Topoisomer −1 was incubated with increasing amounts (at the indicated histone/DNA ratio, rw) of (H3.1–H4)2 (lanes 3_–_5) or (H3.3–H4)2 (lanes 6_–_8) tetramers in the presence of equimolar (to the tetramers) amounts of GST-DAXX. The reaction products were then analyzed on native 4.5% polyacrylamide gel. (Lane 1) Topoisomer −1 DNA. (Lane 2) (H3.3–H4)2 tetrasomes reconstituted on topoisomer −1 by salt dialysis. Positions of the open circular DNA (OC), the naked topoisomer −1 DNA, and the (H3.3–H4)2 tetrasome are indicated.
Figure 5.
Purification of partners associated with e-H3.3 from extracts of stable wild-type and DAXX−/− MEFs. (A) Silver staining of proteins associated with e-H3.3 from soluble nuclear extracts of stable wild-type (lane 1) and DAXX−/− (lane 2) MEFs. H3.3 complexes were purified by double immunoaffinity. The polypeptides identified by mass spectrometry analysis are indicated. Arrows show the positions of partners specific to each complex. (B) Analysis by immunoblotting of proteins associated with e-H3.3 from extracts of stable wild-type (lane 1) and DAXX−/− (lane 2) MEFs using the indicated antibodies. Input fraction (extract) is shown for the blot with anti-HIRA.
Figure 6.
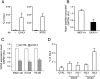
DAXX-dependent deposition of H3.3 on pericentric heterochromatin. (A) DAXX and ATRX are present on pericentric DNA repeats in wild-type MEFs. Presence of DAXX (left panel) and ATRX (right panel) on pericentric DNA repeats was investigated by ChIP assays using specific antibodies. (−Ab) Control sample in which primary antibody was omitted. Results are expressed as percentage of chromatin input used for immunoprecipitation. (B) The level of transcripts from pericentric DNA repeats is reduced in DAXX-deficient cells. Relative mRNA level for pericentric DNA repeats in wild-type and DAXX−/− MEFs was determined by quantitative RT–PCR. Results are represented as relative expression level of pericentric DNA repeats versus GAPDH. Mean ± standard deviation of four independent experiments. (C) Depletion of H3.3A and H3.3B resulted in a decrease in transcription from pericentric DNA repeats. MEFs were transfected with control siRNA (siCTRL) or a mixture of H3.3A and H3.3B siRNA (siH3.3). Relative mRNA levels for pericentric DNA repeats, H3.3A, and H3.3B were determined by quantitative RT–PCR. Results were normalized to GAPDH and were set at 1 in cells transfected with control siRNA. Mean ± standard deviation of three independent experiments. (D) DAXX is required for deposition of H3.3 onto pericentric DNA repeats outside of S phase. DAXX−/− MEFs were deprived of serum for 48 h before being cotransfected with empty vector (CTRL) or else epitope-tagged H3.1 or H3.3 expression vector in combination with DAXX expression vector where indicated. Forty hours later, cells were reinduced for 8 h with 20% FCS in the presence of aphidicolin and were subjected to ChIP assays. Results are expressed as percentage of chromatin input immunoprecipitated. Mean ± standard deviation of three independent experiments.
Figure 7.
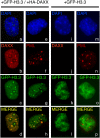
DAXX targets H3.3 to PML-NBs. Resting DAXX−/− MEF cells were transiently transfected with GFP-tagged H3.3 expression vector (+GFP-H3.3) in combination (a–h) or not (i–p) with HA-DAXX expression vector (+HA-DAXX). Forty hours later, cells were supplemented with serum and were paraformaldehyde-fixed after an additional 8 h. Distribution of HA-DAXX (b,j) or endogenous PML (f,n) in GFP-H3.3-positive cells was investigated by immunofluorescence staining using anti-HA or anti-PML antibody, respectively. (a,e,i,m) DNA was stained with DAPI. (d,h,l,p) Merged images correspond to the overlay of red (HA-DAXX or PML) and green fluorescence (GFP-H3.3).
Comment in
- New chaps in the histone chaperone arena.
Campos EI, Reinberg D. Campos EI, et al. Genes Dev. 2010 Jul 1;24(13):1334-8. doi: 10.1101/gad.1946810. Genes Dev. 2010. PMID: 20595228 Free PMC article.
Similar articles
- DAXX-dependent supply of soluble (H3.3-H4) dimers to PML bodies pending deposition into chromatin.
Delbarre E, Ivanauskiene K, Küntziger T, Collas P. Delbarre E, et al. Genome Res. 2013 Mar;23(3):440-51. doi: 10.1101/gr.142703.112. Epub 2012 Dec 5. Genome Res. 2013. PMID: 23222847 Free PMC article. - Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres.
Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Lewis PW, et al. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14075-80. doi: 10.1073/pnas.1008850107. Epub 2010 Jul 22. Proc Natl Acad Sci U S A. 2010. PMID: 20651253 Free PMC article. - H3.Y discriminates between HIRA and DAXX chaperone complexes and reveals unexpected insights into human DAXX-H3.3-H4 binding and deposition requirements.
Zink LM, Delbarre E, Eberl HC, Keilhauer EC, Bönisch C, Pünzeler S, Bartkuhn M, Collas P, Mann M, Hake SB. Zink LM, et al. Nucleic Acids Res. 2017 Jun 2;45(10):5691-5706. doi: 10.1093/nar/gkx131. Nucleic Acids Res. 2017. PMID: 28334823 Free PMC article. - New players in heterochromatin silencing: histone variant H3.3 and the ATRX/DAXX chaperone.
Voon HP, Wong LH. Voon HP, et al. Nucleic Acids Res. 2016 Feb 29;44(4):1496-501. doi: 10.1093/nar/gkw012. Epub 2016 Jan 14. Nucleic Acids Res. 2016. PMID: 26773061 Free PMC article. Review. - A Molecular Prospective for HIRA Complex Assembly and H3.3-Specific Histone Chaperone Function.
Ricketts MD, Marmorstein R. Ricketts MD, et al. J Mol Biol. 2017 Jun 30;429(13):1924-1933. doi: 10.1016/j.jmb.2016.11.010. Epub 2016 Nov 19. J Mol Biol. 2017. PMID: 27871933 Free PMC article. Review.
Cited by
- Histone deposition pathways determine the chromatin landscapes of H3.1 and H3.3 K27M oncohistones.
Sarthy JF, Meers MP, Janssens DH, Henikoff JG, Feldman H, Paddison PJ, Lockwood CM, Vitanza NA, Olson JM, Ahmad K, Henikoff S. Sarthy JF, et al. Elife. 2020 Sep 9;9:e61090. doi: 10.7554/eLife.61090. Elife. 2020. PMID: 32902381 Free PMC article. - Chromatin structure-dependent histone incorporation revealed by a genome-wide deposition assay.
Tachiwana H, Dacher M, Maehara K, Harada A, Seto Y, Katayama R, Ohkawa Y, Kimura H, Kurumizaka H, Saitoh N. Tachiwana H, et al. Elife. 2021 May 10;10:e66290. doi: 10.7554/eLife.66290. Elife. 2021. PMID: 33970102 Free PMC article. - Neurodevelopmental Disorders Caused by Defective Chromatin Remodeling: Phenotypic Complexity Is Highlighted by a Review of ATRX Function.
Timpano S, Picketts DJ. Timpano S, et al. Front Genet. 2020 Aug 11;11:885. doi: 10.3389/fgene.2020.00885. eCollection 2020. Front Genet. 2020. PMID: 32849845 Free PMC article. Review. - Histone chaperone ASF1B promotes human β-cell proliferation via recruitment of histone H3.3.
Paul PK, Rabaglia ME, Wang CY, Stapleton DS, Leng N, Kendziorski C, Lewis PW, Keller MP, Attie AD. Paul PK, et al. Cell Cycle. 2016 Dec;15(23):3191-3202. doi: 10.1080/15384101.2016.1241914. Epub 2016 Oct 18. Cell Cycle. 2016. PMID: 27753532 Free PMC article. - DAXX-dependent supply of soluble (H3.3-H4) dimers to PML bodies pending deposition into chromatin.
Delbarre E, Ivanauskiene K, Küntziger T, Collas P. Delbarre E, et al. Genome Res. 2013 Mar;23(3):440-51. doi: 10.1101/gr.142703.112. Epub 2012 Dec 5. Genome Res. 2013. PMID: 23222847 Free PMC article.
References
- Agez M, Chen J, Guerois R, van Heijenoort C, Thuret JY, Mann C, Ochsenbein F 2007. Structure of the histone chaperone ASF1 bound to the histone H3 C-terminal helix and functional insights. Structure 15: 191–199 - PubMed
- Ahmad K, Henikoff S 2002b. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell 9: 1191–1200 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases