Impaired replication capacity of acute/early viruses in persons who become HIV controllers - PubMed (original) (raw)
. 2010 Aug;84(15):7581-91.
doi: 10.1128/JVI.00286-10. Epub 2010 May 26.
Zabrina L Brumme, Mark A Brockman, Pamela Rosato, Jennifer Sela, Chanson J Brumme, Florencia Pereyra, Daniel E Kaufmann, Alicja Trocha, Brian L Block, Eric S Daar, Elizabeth Connick, Heiko Jessen, Anthony D Kelleher, Eric Rosenberg, Martin Markowitz, Kim Schafer, Florin Vaida, Aikichi Iwamoto, Susan Little, Bruce D Walker
Affiliations
- PMID: 20504921
- PMCID: PMC2897600
- DOI: 10.1128/JVI.00286-10
Impaired replication capacity of acute/early viruses in persons who become HIV controllers
Toshiyuki Miura et al. J Virol. 2010 Aug.
Abstract
Human immunodeficiency virus type 1 (HIV-1) controllers maintain viremia at <2,000 RNA copies/ml without antiretroviral therapy. Viruses from controllers with chronic infection were shown to exhibit impaired replication capacities, in part associated with escape mutations from cytotoxic-T-lymphocyte (CTL) responses. In contrast, little is known about viruses during acute/early infection in individuals who subsequently become HIV controllers. Here, we examine the viral replication capacities, HLA types, and virus sequences from 18 HIV-1 controllers identified during primary infection. gag-protease chimeric viruses constructed using the earliest postinfection samples displayed significantly lower replication capacities than isolates from persons who failed to control viremia (P = 0.0003). Protective HLA class I alleles were not enriched in these early HIV controllers, but viral sequencing revealed a significantly higher prevalence of drug resistance mutations associated with impaired viral fitness in controllers than in noncontrollers (6/15 [40.0%] versus 10/80 [12.5%], P = 0.018). Moreover, of two HLA-B57-positive (B57(+)) controllers identified, both harbored, at the earliest time point tested, signature escape mutations within Gag that likewise impair viral replication capacity. Only five controllers did not express "protective" alleles or harbor viruses with drug resistance mutations; intriguingly, two of them displayed typical B57 signature mutations (T242N), suggesting the acquisition of attenuated viruses from B57(+) donors. These data indicate that acute/early stage viruses from persons who become controllers have evidence of reduced replication capacity during the initial stages of infection which is likely associated with transmitted or acquired CTL escape mutations or transmitted drug resistance mutations. These data suggest that viral dynamics during acute infection have a major impact on HIV disease outcome.
Figures
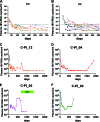
FIG. 1.
Kinetics of plasma virus loads during acute/early phase of infection in individuals who subsequently achieve viremia control (<2,000 RNA copies/ml). (A) Data for six controllers after primary infection (C-PI) who achieved elite control (EC; <50 RNA copies/ml) during the follow-up period. Each line represents the plasma virus loads of an individual patient; the dashed line indicates 2,000 RNA copies/ml. The blue line shows data for C-PI_18, who achieved <50 RNA copies/ml after 600 days post-EDI and, therefore, displayed >50 RNA copies/ml during the period shown in the figure. (B) Data for twelve C-PI who remained viremic during the follow-up period. Each line represents the plasma virus loads of an individual patient; the dashed line indicates 2,000 RNA copies/ml. VC, viremic controllers. (C) Data for C-PI_12, who achieved elite control after 700 days post-EDI after experiencing a blip in viremia. (D) Data for C-PI_04, who achieved elite control after 470 days post-EDI and also experienced virologic escape after 1,500 days post-EDI. (E and F) Data for C-PI_02 and 03, who experienced virologic escape. ART, antiretroviral therapy.
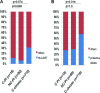
FIG. 2.
Proportions of the individuals expressing protective HLA class I alleles. (A) Proportions of individuals expressing HLA-B57 among controllers after primary infection (C-PI), noncontrollers after primary infection (NC-PI), and chronic HIV controllers (C-chronic). (B) Proportions of individuals expressing protective HLA class I alleles (B13, B27, B51, and B57/B*1516/B*1517/B*5801) among C-PI, NC-PI, and C-chronic.
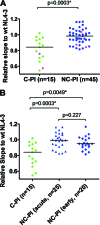
FIG. 3.
Replication capacities of chimeric NL4-3 viruses carrying gag-protease derived from the acute phase of infection in controllers and noncontrollers. Replication capacities were compared between clade B-infected C-PI (n = 15) and NC-PI (n = 45). Average results of duplicate experiments for each virus normalized to the replication capacity of wild-type (wt) NL4-3 virus were plotted (expressed as the slope of the natural log of viral spread between days 3 and 6; see Materials and Methods). (A) Data for all of the chimeric viruses derived from individuals in acute/early phase of infection. The pink dots indicate viruses carrying protease inhibitor (PI) resistance mutations. (B) Data are the same as in the analysis shown in panel A, but the data for viruses derived from the NC-PI are stratified according to phase of infection (acute or early). Horizontal lines show medians of the results.
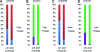
FIG. 4.
Proportions of individuals diagnosed during acute/early phase and carrying viral strains with major drug resistance mutations (see Materials and Methods) are shown. (A) Data for individuals infected with strains with major PI, NRTI, and NNRTI resistance mutations. (B) Data for individuals infected with multiclass drug-resistant strains. (C) Data for individuals without protective HLA class I alleles infected with strains with major PI, NRTI, and NNRTI resistance mutations. (D) Data for individuals without protective HLA class I alleles infected with multiclass drug-resistant strains.
Similar articles
- HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition.
Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, Rothchild AC, Li B, Trocha A, Cutrell E, Frahm N, Brander C, Toth I, Arts EJ, Allen TM, Walker BD. Miura T, et al. J Virol. 2009 Mar;83(6):2743-55. doi: 10.1128/JVI.02265-08. Epub 2008 Dec 30. J Virol. 2009. PMID: 19116253 Free PMC article. - Impaired Nef function is associated with early control of HIV-1 viremia.
Kuang XT, Li X, Anmole G, Mwimanzi P, Shahid A, Le AQ, Chong L, Qian H, Miura T, Markle T, Baraki B, Connick E, Daar ES, Jessen H, Kelleher AD, Little S, Markowitz M, Pereyra F, Rosenberg ES, Walker BD, Ueno T, Brumme ZL, Brockman MA. Kuang XT, et al. J Virol. 2014 Sep 1;88(17):10200-13. doi: 10.1128/JVI.01334-14. Epub 2014 Jun 25. J Virol. 2014. PMID: 24965469 Free PMC article. - Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated for in chronic infection.
Brockman MA, Brumme ZL, Brumme CJ, Miura T, Sela J, Rosato PC, Kadie CM, Carlson JM, Markle TJ, Streeck H, Kelleher AD, Markowitz M, Jessen H, Rosenberg E, Altfeld M, Harrigan PR, Heckerman D, Walker BD, Allen TM. Brockman MA, et al. J Virol. 2010 Nov;84(22):11937-49. doi: 10.1128/JVI.01086-10. Epub 2010 Sep 1. J Virol. 2010. PMID: 20810731 Free PMC article. - HIV-1 replicative fitness in elite controllers.
Lobritz MA, Lassen KG, Arts EJ. Lobritz MA, et al. Curr Opin HIV AIDS. 2011 May;6(3):214-20. doi: 10.1097/COH.0b013e3283454cf5. Curr Opin HIV AIDS. 2011. PMID: 21430530 Free PMC article. Review. - [HIV controllers: how these patients control viral replication?].
Lambotte O. Lambotte O. Med Sci (Paris). 2012 Feb;28(2):172-8. doi: 10.1051/medsci/2012282015. Epub 2012 Feb 27. Med Sci (Paris). 2012. PMID: 22377305 Review. French.
Cited by
- The implications of viral reservoirs on the elite control of HIV-1 infection.
Buckheit RW 3rd, Salgado M, Martins KO, Blankson JN. Buckheit RW 3rd, et al. Cell Mol Life Sci. 2013 Mar;70(6):1009-19. doi: 10.1007/s00018-012-1101-7. Epub 2012 Aug 4. Cell Mol Life Sci. 2013. PMID: 22864624 Free PMC article. Review. - Transmitted virus fitness and host T cell responses collectively define divergent infection outcomes in two HIV-1 recipients.
Yue L, Pfafferott KJ, Baalwa J, Conrod K, Dong CC, Chui C, Rong R, Claiborne DT, Prince JL, Tang J, Ribeiro RM, Cormier E, Hahn BH, Perelson AS, Shaw GM, Karita E, Gilmour J, Goepfert P, Derdeyn CA, Allen SA, Borrow P, Hunter E. Yue L, et al. PLoS Pathog. 2015 Jan 8;11(1):e1004565. doi: 10.1371/journal.ppat.1004565. eCollection 2015 Jan. PLoS Pathog. 2015. PMID: 25569444 Free PMC article. - Contribution of the HIV-1 Envelope Glycoprotein to AIDS Pathogenesis and Clinical Progression.
Valenzuela-Fernández A, Cabrera-Rodríguez R, Casado C, Pérez-Yanes S, Pernas M, García-Luis J, Marfil S, Olivares I, Estévez-Herrera J, Trujillo-González R, Blanco J, Lopez-Galindez C. Valenzuela-Fernández A, et al. Biomedicines. 2022 Sep 2;10(9):2172. doi: 10.3390/biomedicines10092172. Biomedicines. 2022. PMID: 36140273 Free PMC article. Review. - High viral fitness during acute HIV-1 infection.
Arnott A, Jardine D, Wilson K, Gorry PR, Merlin K, Grey P, Law MG, Dax EM, Kelleher AD, Smith DE, McPhee DA; Pulse Study Team. Arnott A, et al. PLoS One. 2010 Sep 9;5(9):e12631. doi: 10.1371/journal.pone.0012631. PLoS One. 2010. PMID: 20844589 Free PMC article. - HIV controllers: a multifactorial phenotype of spontaneous viral suppression.
Thèze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. Thèze J, et al. Clin Immunol. 2011 Oct;141(1):15-30. doi: 10.1016/j.clim.2011.07.007. Epub 2011 Aug 4. Clin Immunol. 2011. PMID: 21865089 Free PMC article. Review.
References
- Altfeld, M., M. M. Addo, E. S. Rosenberg, F. M. Hecht, P. K. Lee, M. Vogel, X. G. Yu, R. Draenert, M. N. Johnston, D. Strick, T. M. Allen, M. E. Feeney, J. O. Kahn, R. P. Sekaly, J. A. Levy, J. K. Rockstroh, P. J. Goulder, and B. D. Walker. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581-2591. - PubMed
- Bailey, J. R., K. G. Lassen, H. C. Yang, T. C. Quinn, S. C. Ray, J. N. Blankson, and R. F. Siliciano. 2006. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 80:4758-4770. - PMC - PubMed
- Bailey, J. R., H. Zhang, B. W. Wegweiser, H. C. Yang, L. Herrera, A. Ahonkhai, T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2007. Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J. Infect. Dis. 196:50-55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI028568/AI/NIAID NIH HHS/United States
- AI041534/AI/NIAID NIH HHS/United States
- AI047033/AI/NIAID NIH HHS/United States
- CAPMC/ CIHR/Canada
- HHMI/Howard Hughes Medical Institute/United States
- AI030914/AI/NIAID NIH HHS/United States
- U01 AI041534/AI/NIAID NIH HHS/United States
- R37 AI028568/AI/NIAID NIH HHS/United States
- R01 HL092565/HL/NHLBI NIH HHS/United States
- P01 AI055356/AI/NIAID NIH HHS/United States
- R01 AI047033/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States
- R01 AI028568/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- R01 AI030914/AI/NIAID NIH HHS/United States
- AI55356/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials