TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype - PubMed (original) (raw)
. 2010 Nov;225(3):682-91.
doi: 10.1002/jcp.22264.
Pasquale Sansone, Sara Mari, Gabriele D'Uva, Simona Tavolari, Tiziana Guarnieri, Mario Taffurelli, Claudio Ceccarelli, Donatella Santini, Pasquale Chieco, Kenneth B Marcu, Massimiliano Bonafè
Affiliations
- PMID: 20509143
- PMCID: PMC2939957
- DOI: 10.1002/jcp.22264
TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype
Gianluca Storci et al. J Cell Physiol. 2010 Nov.
Abstract
Extracellular and intracellular mediators of inflammation, such as tumor necrosis factor alpha (TNFα) and NF-kappaB (NF-κB), play major roles in breast cancer pathogenesis, progression and relapse. SLUG, a mediator of the epithelial-mesenchymal transition process, is over-expressed in CD44(+)/CD24(-) tumor initiating breast cancer cells and in basal-like carcinoma, a subtype of aggressive breast cancer endowed with a stem cell-like gene expression profile. Cancer stem cells also over-express members of the pro-inflammatory NF-κB network, but their functional relationship with SLUG expression in breast cancer cells remains unclear. Here, we show that TNFα treatment of human breast cancer cells up-regulates SLUG with a dependency on canonical NF-κB/HIF1α signaling, which is strongly enhanced by p53 inactivation. Moreover, SLUG up-regulation engenders breast cancer cells with stem cell-like properties including enhanced expression of CD44 and Jagged-1 in conjunction with estrogen receptor alpha down-regulation, growth as mammospheres, and extracellular matrix invasiveness. Our results reveal a molecular mechanism whereby TNFα, a major pro-inflammatory cytokine, imparts breast cancer cells with stem cell-like features, which are connected to increased tumor aggressiveness.
© 2010 Wiley-Liss, Inc.
Conflict of interest statement
Conflit of interest: All authors declare no conflict of interest.
Figures
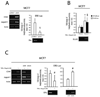
FIGURE 1. TNFα up-regulates CD44 and Jagged-1 and down-regulates ERα expression via SLUG
(A): RT-PCR analysis of CD44, Jagged-1 and ERα mRNA level, and ERE-Luc in MCF7 cells transiently transfected with empty (pcDNA3.1) and human SLUG encoding (pSLUG) vector (1µg each, 24h); (B): NF-κB-Luc, SLUG promoter driven luciferase activity assay (SLUG-Luc) and RT-PCR analysis of SLUG mRNA in MCF7 cells exposed to TNFα (10ng/ml, 24h); (C): CD44, Jagged-1 and ERα mRNA level and ERE-Luc assay in MCF7 cells transiently transfected with SCR or SLUG specific siRNA (1µg, 48h) exposed or less to TNFα (10ng/ml, 24h). Data are presented as mean +/− S.D. of three replicates, p values of unpaired t tests: *<0.05, #<0.01 and § <0.005. β-Actin was used as reference control (RT-PCR analysis in panel A and B are normalized on the same β-Actin).
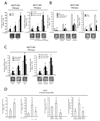
FIGURE 2. TNFα/NF-κB signalling promotes MS formation and invasive capacity of breast cancer cells via SLUG up-regulation
(A): MS forming capacity of empty/pSLUG transiently transfected (1µg, 24h) MCF7 cells and pCtoGMB/ shSLUG stably transduced MCF7 cells exposed or less to TNFα (10 ng/ml, 24h); (B): MS forming capacity of MCF7 cells exposed to the IκBα degradation inhibitors Parthenolide or Bay 11-7082 (5µM, 24h each), the specific IKKβ inhibitor sc-514 (5µM, 24h each), or stably transduced with an IKKβ specific shRNA/empty expressing retroviral vector; (C): MS forming capacity of pCtoGMB/shSLUG MCF7 cells transduced with empty or p65/IKKβ-CA encoding vector, representative MS pictures are also reported. The scale bar inset corresponds to 100µm; (D): Invasion assay in pSLUG transfected (1µg), TNFα exposed (10ng/ml, 24h), p65/IKKβ-CA transduced pCtoGMB/shSLUG MCF7 cells. Data are presented as mean +/− S.D. of three replicates, p values of unpaired t tests: *<0.05, #<0.01 and § <0.005. The scale bar represents 100 µm.
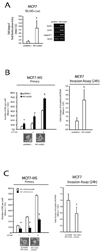
FIGURE 3. HIF1α is necessary for the SLUG-dependent stem cell-like gene signature, MS formation and invasive capacity of breast cancer cells
(A): SLUG-Luc activity assay and RT-PCR analysis of SLUG, CD44 and Jagged-1 mRNA level in MCF7 cells transiently transfected with pCDNA3.1 and HIF1α encoding vector (HIF1αODD, 1µg, 24h); (B): MS forming and invasive capacity assay of MCF7 cells transiently transfected with HIF1αODD (1µg); (C): MS forming and invasive capacity assay of pCtoGMB/shSLUG MCF7 cells transiently transfected with HIF1αODD (1µg). Representative MS pictures are also reported. Data are presented as mean +/− S.D. of three replicates, p values of unpaired t tests *<0.05; #<0.01; §<0.005. The scale bar represents 100µm. β-Actin was used as reference control.
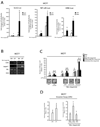
FIGURE 4. p53 compromised cells show an over-activation of the NF-κB/HIF1α axis and SLUG expression in response to TNFα
(A): SLUG-Luc, NF-κB-Luc and HRE-Luc activity assays in pBabe/p53 dominant negative (p53D) transduced MCF7 cells exposed or less to TNFα 10 ng/ml, 24h; (B): RT-PCR analysis of CD44, Jagged-1, ERα in pBabe/p53D and pBabe/p53D exposed or less to TNFα 10ng/ml, 24h; (C): MS forming capacity of pBabe/p53D and pCtoGMB/shSLUG transduced MCF7 cells exposed or less to TNFα (10 ng/ml, 24h); representative MS pictures are also reported (D): Invasion assay of pBabe/p53D and pCtoGMB/shSLUG transduced MCF7 cells exposed or less to TNFα 0 (10 ng/ml, 24h). Data are presented as mean +/− S.D. of three replicates, p values of unpaired t tests: *<0.05; #<0.01 and §<0.005. Reference scale bar is 10µm. β-Actin was used as reference control.
FIGURE 5
Inflammatory environment activation of NF-kappaB/HIF1α/SLUG/β-Catenin axis drives the up-regulation of the basal/stem cell-like gene expression profile in breast cancer cell. p53 loss of function up-regulates the outlined interplay.
Similar articles
- SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype.
Bhat-Nakshatri P, Appaiah H, Ballas C, Pick-Franke P, Goulet R Jr, Badve S, Srour EF, Nakshatri H. Bhat-Nakshatri P, et al. BMC Cancer. 2010 Aug 6;10:411. doi: 10.1186/1471-2407-10-411. BMC Cancer. 2010. PMID: 20691079 Free PMC article. - Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner.
Shao S, Zhao X, Zhang X, Luo M, Zuo X, Huang S, Wang Y, Gu S, Zhao X. Shao S, et al. Mol Cancer. 2015 Feb 3;14(1):28. doi: 10.1186/s12943-015-0295-3. Mol Cancer. 2015. PMID: 25645291 Free PMC article. - NF-κB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype.
Yamamoto M, Taguchi Y, Ito-Kureha T, Semba K, Yamaguchi N, Inoue J. Yamamoto M, et al. Nat Commun. 2013;4:2299. doi: 10.1038/ncomms3299. Nat Commun. 2013. PMID: 23934482 - Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y. Chen X, et al. Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review. - NF-κB, stem cells and breast cancer: the links get stronger.
Shostak K, Chariot A. Shostak K, et al. Breast Cancer Res. 2011 Jul 26;13(4):214. doi: 10.1186/bcr2886. Breast Cancer Res. 2011. PMID: 21867572 Free PMC article. Review.
Cited by
- Slug Is Increased in Vascular Remodeling and Induces a Smooth Muscle Cell Proliferative Phenotype.
Coll-Bonfill N, Peinado VI, Pisano MV, Párrizas M, Blanco I, Evers M, Engelmann JC, García-Lucio J, Tura-Ceide O, Meister G, Barberà JA, Musri MM. Coll-Bonfill N, et al. PLoS One. 2016 Jul 21;11(7):e0159460. doi: 10.1371/journal.pone.0159460. eCollection 2016. PLoS One. 2016. PMID: 27441378 Free PMC article. - Targeting the KLF5-EphA2 axis can restrain cancer stemness and overcome chemoresistance in basal-like breast cancer.
Zhao P, Sun J, Huang X, Zhang X, Liu X, Liu R, Du G, Gan W, Yang C, Tang Y, Chen C, Jiang D. Zhao P, et al. Int J Biol Sci. 2023 Mar 21;19(6):1861-1874. doi: 10.7150/ijbs.82567. eCollection 2023. Int J Biol Sci. 2023. PMID: 37063424 Free PMC article. - Epigenetic Regulation of Inflammatory Cytokine-Induced Epithelial-To-Mesenchymal Cell Transition and Cancer Stem Cell Generation.
Markopoulos GS, Roupakia E, Marcu KB, Kolettas E. Markopoulos GS, et al. Cells. 2019 Sep 25;8(10):1143. doi: 10.3390/cells8101143. Cells. 2019. PMID: 31557902 Free PMC article. Review. - CD200 mimetic aptamer PEG-M49 markedly increases the therapeutic effects of pegylated liposomal doxorubicin in a mouse model of metastatic breast carcinoma: an effect independent of CD200 receptor 1.
Erin N, Dilmaç S, Curry A, Duymuş Ö, Tanriover G, Prodeus A, Gariepy J, Gorczynski RM. Erin N, et al. Cancer Immunol Immunother. 2020 Jan;69(1):103-114. doi: 10.1007/s00262-019-02444-3. Epub 2019 Dec 6. Cancer Immunol Immunother. 2020. PMID: 31811336 Free PMC article. - Desmoglein 3 Silencing Inhibits Inflammation and Goblet Cell Mucin Secretion in a Mouse Model of Chronic Rhinosinusitis via Disruption of the Wnt/β-Catenin Signaling Pathway.
Cheng J, Yang J, Xue K, Zhao Y, Zhao C, Li S, Wang Z. Cheng J, et al. Inflammation. 2019 Aug;42(4):1370-1382. doi: 10.1007/s10753-019-00998-z. Inflammation. 2019. PMID: 31028575
References
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. Review. - PubMed
- Bertucci F, et al. How different are luminal A and basal breast cancers? Int J Cancer. 2009;124:1338–1348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous