Induction of neuro-protective/regenerative genes in stem cells infiltrating post-ischemic brain tissue - PubMed (original) (raw)
Induction of neuro-protective/regenerative genes in stem cells infiltrating post-ischemic brain tissue
Gokhan Yilmaz et al. Exp Transl Stroke Med. 2010.
Abstract
Background: Although the therapeutic potential of bone marrow-derived stromal stem cells (BMSC) has been demonstrated in different experimental models of ischemic stroke, it remains unclear how stem cells (SC) induce neuroprotection following stroke. In this study, we describe a novel method for isolating BMSC that infiltrate postischemic brain tissue and use this method to identify the genes that are persistently activated or depressed in BMSC that infiltrate brain tissue following ischemic stroke.
Methods: Ischemic strokes were induced in C57BL/6 mice by middle cerebral artery occlusion for 1 h, followed by reperfusion. BMSC were isolated from H-2 Kb-tsA58 (immortomouse) mice, and were administered (i.v.) 24 h after reperfusion. At the peak of therapeutic improvement (14 days after the ischemic insult), infarcted brain tissue was isolated, and the BMSC were isolated by culturing at 33 degrees C. Microarray analysis and RT-PCR were performed to compare differential gene expression between naïve and infiltrating BMSC populations.
Results: Z-scoring revealed dramatic differences in the expression of extracellular genes between naïve and infiltrating BMSC. Pair-wise analysis detected 80 extracellular factor genes that were up-regulated (>/= 2 fold, P < 0.05, Benjamini-Hochberg correction) between naïve and infiltrated BMSC. Although several anticipated neuroregenerative, nerve guidance and angiogenic factor (e.g., bFGF, bone morphogenetic protein, angiopoietins, neural growth factor) genes exhibited an increased expression, a remarkable induction of genes for nerve guidance survival (e.g., cytokine receptor-like factor 1, glypican 1, Dickkopf homolog 2, osteopontin) was also noted.
Conclusions: BMSC infiltrating the post-ischemic brain exhibit persistent epigenetic changes in gene expression for numerous extracellular genes, compared to their naïve counterparts. These genes are relevant to the neuroprotection, regeneration and angiogenesis previously described following stem cell therapy in animal models of ischemic stroke.
Figures
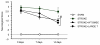
Figure 1
Neurological scores of sham, stroke, stroke +WT BMSC and stroke + Large T BMSC groups over a two week period after stroke or sham surgery. * represents significant difference from sham group (p < 0.05), # represents significant difference from stroke group (P < 0.05), one-way ANOVA with Tukey's post-hoc test.
Figure 2
Large T BMSC isolated from ischemic brain. A. Nuclear DAPI Staining, B. Large T immunostaining of the same sample.
Figure 3
Volcano graph of gene expression changes in BMSC isolated from ischemic brain (Group1) versus naïve BMSC (Group2).
Figure 4
Fold-change in gene expression detected by RT-PCR versus microarray.
Figure 5
Z-scores of gene ontology groups that changed in BMSC isolated from ischemic brain.
Figure 6
Changes in KEGG pathways in BMSC isolated from ischemic brain. Changes filtered for parameters Z-score > 2 and 10 or more changed genes in related group.
Figure 7
Axon guidance KEGG pathway affected in BMSC isolated from ischemic brain. Genes affected represented in red.
Similar articles
- Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy.
Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, Iwama T. Ikegame Y, et al. Cytotherapy. 2011 Jul;13(6):675-85. doi: 10.3109/14653249.2010.549122. Epub 2011 Jan 13. Cytotherapy. 2011. PMID: 21231804 - Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke.
Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Cui X, et al. Stem Cells. 2007 Nov;25(11):2777-85. doi: 10.1634/stemcells.2007-0169. Epub 2007 Jul 19. Stem Cells. 2007. PMID: 17641243 Free PMC article. - Sodium ferulate and n-butylidenephthalate combined with bone marrow stromal cells (BMSCs) improve the therapeutic effects of angiogenesis and neurogenesis after rat focal cerebral ischemia.
Zhang Q, Zhao Y, Xu Y, Chen Z, Liu N, Ke C, Liu B, Wu W. Zhang Q, et al. J Transl Med. 2016 Jul 28;14(1):223. doi: 10.1186/s12967-016-0979-5. J Transl Med. 2016. PMID: 27465579 Free PMC article. - Bone marrow stem cells regenerate infarcted myocardium.
Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Orlic D, et al. Pediatr Transplant. 2003;7 Suppl 3:86-8. doi: 10.1034/j.1399-3046.7.s3.13.x. Pediatr Transplant. 2003. PMID: 12603699 Review. - Regenerative therapy for stroke.
Chang YC, Shyu WC, Lin SZ, Li H. Chang YC, et al. Cell Transplant. 2007;16(2):171-81. Cell Transplant. 2007. PMID: 17474298 Review.
Cited by
- Impaired Peripheral Vascular Function Following Ischemic Stroke in Mice: Potential Insights into Blood Pressure Variations in the Post-Stroke Patient.
Yilmaz G, Alexander JS. Yilmaz G, et al. Pathophysiology. 2024 Sep 5;31(3):488-501. doi: 10.3390/pathophysiology31030036. Pathophysiology. 2024. PMID: 39311310 Free PMC article. - FAM19A5 Deficiency Mitigates the Aβ Plaque Burden and Improves Cognition in Mouse Models of Alzheimer's Disease.
Park S, Shahapal A, Yoo S, Kwak H, Lee M, Lee SM, Hwang JI, Seong JY. Park S, et al. Exp Neurobiol. 2024 Aug 31;33(4):193-201. doi: 10.5607/en24017. Exp Neurobiol. 2024. PMID: 39266475 Free PMC article. - Human placenta mesenchymal stem cell protection in ischemic stroke is angiotensin converting enzyme-2 and masR receptor-dependent.
Barzegar M, Vital S, Stokes KY, Wang Y, Yun JW, White LA, Chernyshev O, Kelley RE, Alexander JS. Barzegar M, et al. Stem Cells. 2021 Oct;39(10):1335-1348. doi: 10.1002/stem.3426. Epub 2021 Jun 22. Stem Cells. 2021. PMID: 34124808 Free PMC article. - Mesenchymal Stem Cell Derived Extracellular Vesicles for Repairing the Neurovascular Unit after Ischemic Stroke.
Davis C, Savitz SI, Satani N. Davis C, et al. Cells. 2021 Mar 31;10(4):767. doi: 10.3390/cells10040767. Cells. 2021. PMID: 33807314 Free PMC article. Review. - Emergence of signs of neural cells after exposure of bone marrow-derived mesenchymal stem cells to fetal brain extract.
Jahromi IR, Mehrabani D, Mohammadi A, Seno MM, Dianatpour M, Zare S, Tamadon A. Jahromi IR, et al. Iran J Basic Med Sci. 2017 Mar;20(3):301-307. doi: 10.22038/ijbms.2017.8360. Iran J Basic Med Sci. 2017. PMID: 28392903 Free PMC article.
References
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. - PubMed
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials