Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study - PubMed (original) (raw)
. 2010 May 29;375(9729):1896-905.
doi: 10.1016/S0140-6736(10)60357-1.
Amy C H Lee, Marjorie Robbins, Joan B Geisbert, Anna N Honko, Vandana Sood, Joshua C Johnson, Susan de Jong, Iran Tavakoli, Adam Judge, Lisa E Hensley, Ian Maclachlan
Affiliations
- PMID: 20511019
- PMCID: PMC7138079
- DOI: 10.1016/S0140-6736(10)60357-1
Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study
Thomas W Geisbert et al. Lancet. 2010.
Abstract
Background: We previously showed that small interfering RNAs (siRNAs) targeting the Zaire Ebola virus (ZEBOV) RNA polymerase L protein formulated in stable nucleic acid-lipid particles (SNALPs) completely protected guineapigs when administered shortly after a lethal ZEBOV challenge. Although rodent models of ZEBOV infection are useful for screening prospective countermeasures, they are frequently not useful for prediction of efficacy in the more stringent non-human primate models. We therefore assessed the efficacy of modified non-immunostimulatory siRNAs in a uniformly lethal non-human primate model of ZEBOV haemorrhagic fever.
Methods: A combination of modified siRNAs targeting the ZEBOV L polymerase (EK-1 mod), viral protein (VP) 24 (VP24-1160 mod), and VP35 (VP35-855 mod) were formulated in SNALPs. A group of macaques (n=3) was given these pooled anti-ZEBOV siRNAs (2 mg/kg per dose, bolus intravenous infusion) after 30 min, and on days 1, 3, and 5 after challenge with ZEBOV. A second group of macaques (n=4) was given the pooled anti-ZEBOV siRNAs after 30 min, and on days 1, 2, 3, 4, 5, and 6 after challenge with ZEBOV.
Findings: Two (66%) of three rhesus monkeys given four postexposure treatments of the pooled anti-ZEBOV siRNAs were protected from lethal ZEBOV infection, whereas all macaques given seven postexposure treatments were protected. The treatment regimen in the second study was well tolerated with minor changes in liver enzymes that might have been related to viral infection.
Interpretation: This complete postexposure protection against ZEBOV in non-human primates provides a model for the treatment of ZEBOV-induced haemorrhagic fever. These data show the potential of RNA interference as an effective postexposure treatment strategy for people infected with Ebola virus, and suggest that this strategy might also be useful for treatment of other emerging viral infections.
Funding: Defense Threat Reduction Agency.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Figure 1
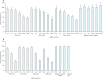
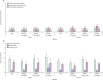
Effect of small interfering RNAs (siRNAs) targeting virion protein genes VP24 (A) and VP35 (B) of Zaire Ebola virus (ZEBOV) expressed in a non-viral plasmid-based system in HepG2 cells Error bars represent SD of triplicate tissue culture wells.
Figure 2
Effect of small interfering RNAs (siRNAs) on synthesis of interleukin 6 (A) and interferon α (B), induction of interferon-induced protein with tetratricopeptide repeats (IFIT1) mRNA in mice (C), and induction of interferon α in human peripheral blood mononuclear cell cultures (D) Data are mean (SD). Error bars represent SD. Luc=luciferase. PBS=phosphate-buffered saline. Luc mod=modified luciferase. GAPDH=glyceraldehyde 3-phosphate dehydrogenase.
Figure 3
Rapid amplification of cDNA ends (RACE)-PCR of small interfering RNA (siRNA)-mediated cleavage of Zaire Ebola virus (ZEBOV) L polymerase, virion protein (VP) 24 (VP24), and VP35 mRNAs in ZEBOV-infected Vero E6 cells Vero E6 cells were treated with stable nucleic acid-lipid particles (SNALPs) containing ZEBOV siRNA cocktail, modified luciferase (Luc mod), or phosphate-buffered saline (PBS). (B) Vero E6 cells were treated with SNALPs containing EK-1-mod, VP24-1160-mod, VP35-855-mod, ZEBOV siRNA cocktail, Luc mod, or PBS. The order of samples for each RACE PCR shown in the gel is SNALPs containing PBS, single gene-specific siRNAs (EK-1-mod, VP24-1160-mod, or VP35-855-mod), ZEBOV cocktail, and Luc mod. Lanes 1 and 17 are the 100 base pair (bp) ladder, lanes 2–5 are EK-1 RACE PCR, lanes 7–10 are VP24-1160 RACE, and lanes 12–15 are VP35-855 RACE. Lanes 6, 11, and 16 are empty.
Figure 4
Proportion of differentiated cells with uptake of stable nucleic acid-lipid particles (SNALPs) containing fluorescein-isothiocyanate-labelled modified luciferase small interfering RNAs
Figure 5
Effect of daily administration of stable nucleic acid-lipid particles (SNALPs) containing Zaire Ebola virus (ZEBOV) small interfering RNAs on activities (siRNAs) of alanine aminotransferase, aspartate aminotransferase, and sorbitol dehydrogenase (A), and blood cell counts (B) in mice PBS=phosphate-buffered saline.
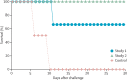
Figure 6
Survival curves for Zaire Ebola virus (ZEBOV)-infected rhesus macaques treated after challenge with stable nucleic acid-lipid particles containing ZEBOV small interfering RNAs Study 1: four postexposure treatments. Study 2: seven post exposure treatments.
Comment in
- Are we any closer to combating Ebola infections?
Feldmann H. Feldmann H. Lancet. 2010 May 29;375(9729):1850-2. doi: 10.1016/S0140-6736(10)60597-1. Lancet. 2010. PMID: 20511001 Free PMC article. No abstract available.
Similar articles
- Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference.
Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee AC, Judge A, Jeffs LB, MacLachlan I. Geisbert TW, et al. J Infect Dis. 2006 Jun 15;193(12):1650-7. doi: 10.1086/504267. Epub 2006 May 10. J Infect Dis. 2006. PMID: 16703508 Free PMC article. - Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever.
Marzi A, Yoshida R, Miyamoto H, Ishijima M, Suzuki Y, Higuchi M, Matsuyama Y, Igarashi M, Nakayama E, Kuroda M, Saijo M, Feldmann F, Brining D, Feldmann H, Takada A. Marzi A, et al. PLoS One. 2012;7(4):e36192. doi: 10.1371/journal.pone.0036192. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558378 Free PMC article. - Passive Immunotherapy: Assessment of Convalescent Serum Against Ebola Virus Makona Infection in Nonhuman Primates.
Mire CE, Geisbert JB, Agans KN, Thi EP, Lee AC, Fenton KA, Geisbert TW. Mire CE, et al. J Infect Dis. 2016 Oct 15;214(suppl 3):S367-S374. doi: 10.1093/infdis/jiw333. Epub 2016 Aug 28. J Infect Dis. 2016. PMID: 27571900 Free PMC article. - [Recent Advances in Vaccines and Drugs Against the Ebola Virus].
Zhu X, Yao C, Wei Y, Kou Z, Hu K. Zhu X, et al. Bing Du Xue Bao. 2015 May;31(3):287-92. Bing Du Xue Bao. 2015. PMID: 26470536 Review. Chinese. - Correlates of vaccine-induced protective immunity against Ebola virus disease.
Medaglini D, Santoro F, Siegrist CA. Medaglini D, et al. Semin Immunol. 2018 Oct;39:65-72. doi: 10.1016/j.smim.2018.07.003. Epub 2018 Jul 21. Semin Immunol. 2018. PMID: 30041831 Review.
Cited by
- Pharmacokinetic Behaviors of Intravenously Administered siRNA in Glandular Tissues.
Huang Y, Cheng Q, Ji JL, Zheng S, Du L, Meng L, Wu Y, Zhao D, Wang X, Lai L, Cao H, Xiao K, Gao S, Liang Z. Huang Y, et al. Theranostics. 2016 Jun 18;6(10):1528-41. doi: 10.7150/thno.15246. eCollection 2016. Theranostics. 2016. PMID: 27446488 Free PMC article. - Ebola virus disease and critical illness.
Leligdowicz A, Fischer WA 2nd, Uyeki TM, Fletcher TE, Adhikari NK, Portella G, Lamontagne F, Clement C, Jacob ST, Rubinson L, Vanderschuren A, Hajek J, Murthy S, Ferri M, Crozier I, Ibrahima E, Lamah MC, Schieffelin JS, Brett-Major D, Bausch DG, Shindo N, Chan AK, O'Dempsey T, Mishra S, Jacobs M, Dickson S, Lyon GM 3rd, Fowler RA. Leligdowicz A, et al. Crit Care. 2016 Jul 29;20(1):217. doi: 10.1186/s13054-016-1325-2. Crit Care. 2016. PMID: 27468829 Free PMC article. Review. - Ebola virus outbreak, updates on current therapeutic strategies.
Elshabrawy HA, Erickson TB, Prabhakar BS. Elshabrawy HA, et al. Rev Med Virol. 2015 Jul;25(4):241-53. doi: 10.1002/rmv.1841. Epub 2015 May 11. Rev Med Virol. 2015. PMID: 25962887 Free PMC article. Review. - Lipid nanoparticle-mediated siRNA delivery for safe targeting of human CML in vivo.
Jyotsana N, Sharma A, Chaturvedi A, Budida R, Scherr M, Kuchenbauer F, Lindner R, Noyan F, Sühs KW, Stangel M, Grote-Koska D, Brand K, Vornlocher HP, Eder M, Thol F, Ganser A, Humphries RK, Ramsay E, Cullis P, Heuser M. Jyotsana N, et al. Ann Hematol. 2019 Aug;98(8):1905-1918. doi: 10.1007/s00277-019-03713-y. Epub 2019 May 18. Ann Hematol. 2019. PMID: 31104089 Free PMC article. - Animal models for Ebola and Marburg virus infections.
Nakayama E, Saijo M. Nakayama E, et al. Front Microbiol. 2013 Sep 5;4:267. doi: 10.3389/fmicb.2013.00267. Front Microbiol. 2013. PMID: 24046765 Free PMC article. Review.
References
- Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, editors. Fields virology. Lippincott Williams and Wilkins; Philadelphia: 2006. pp. 1409–1448.
- Sanchez A, Kiley MP, Holloway BP, Auperin DD. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993;29:215–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials