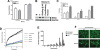Multiple effects of silymarin on the hepatitis C virus lifecycle - PubMed (original) (raw)
. 2010 Jun;51(6):1912-21.
doi: 10.1002/hep.23587.
Amina Negash, Olivia J Kane, Laura E Martinez, Yaakov Nahmias, Nigel Bourne, David M Owen, Joe Grove, Claire Brimacombe, Jane A McKeating, Eve-Isabelle Pécheur, Tyler N Graf, Nicholas H Oberlies, Volker Lohmann, Feng Cao, John E Tavis, Stephen J Polyak
Affiliations
- PMID: 20512985
- PMCID: PMC2909978
- DOI: 10.1002/hep.23587
Multiple effects of silymarin on the hepatitis C virus lifecycle
Jessica Wagoner et al. Hepatology. 2010 Jun.
Abstract
Silymarin, an extract from milk thistle (Silybum marianum), and its purified flavonolignans have been recently shown to inhibit hepatitis C virus (HCV) infection, both in vitro and in vivo. In the current study, we further characterized silymarin's antiviral actions. Silymarin had antiviral effects against hepatitis C virus cell culture (HCVcc) infection that included inhibition of virus entry, RNA and protein expression, and infectious virus production. Silymarin did not block HCVcc binding to cells but inhibited the entry of several viral pseudoparticles (pp), and fusion of HCVpp with liposomes. Silymarin but not silibinin inhibited genotype 2a NS5B RNA-dependent RNA polymerase (RdRp) activity at concentrations 5 to 10 times higher than required for anti-HCVcc effects. Furthermore, silymarin had inefficient activity on the genotype 1b BK and four 1b RDRPs derived from HCV-infected patients. Moreover, silymarin did not inhibit HCV replication in five independent genotype 1a, 1b, and 2a replicon cell lines that did not produce infectious virus. Silymarin inhibited microsomal triglyceride transfer protein activity, apolipoprotein B secretion, and infectious virion production into culture supernatants. Silymarin also blocked cell-to-cell spread of virus.
Conclusion: Although inhibition of in vitro NS5B polymerase activity is demonstrable, the mechanisms of silymarin's antiviral action appear to include blocking of virus entry and transmission, possibly by targeting the host cell.
Figures
Figure 1. Antiviral effects of silymarin against HCVcc
A, Silymarin inhibits virus infection in multiple HCVcc systems. Huh-7.5 cells were incubated with 80μM silymarin or DMSO for 1hr at 37C before inoculation with either H77/JFH or J6/JFH HCVcc viruses. The infection was allowed to proceed in the presence of the compounds for 48 and 72hrs before virus was quantitated by staining for NS5A-positive foci. Percent inhibition reflects inhibition of NS5A-positive foci by silymarin relative to the DMSO control. B, Silymarin does not block virus binding. Huh7.5.1 cells were incubated at 4°C for 5 hours in the presence of HCVcc (JFH-1, m.o.i. of 0.01) with or without 40μM silymarin. Cells were then washed extensively at 4°C and then cells were incubated for 72 hours at 37°C to allow the HCV lifecycle to continue in the presence or absence of additional silymarin. “M” denotes mock infected cells. “0” denotes cells that were infected but not treated with anything. “Binding @ 4C” denotes silymarin was only present during the 5-hour adsorption period. “Post-binding” denotes silymarin was added after the 5-hour adsorption period and for the duration of the infection. “Normal” denotes silymarin was present during the 5-hour adsorption and for the duration of the infection. C, Silymarin inhibits pseudoparticle entry. Huh-7.5 cells were treated with silymarin (SM) or equivalent volume of DMSO for 1 hour prior to infection with HCV, VSV, or MLV pseudoparticles (pp). 72 h post-infection, the medium was removed and luciferase activity was measured on cell lysates. D, Silymarin blocks HCVpp-mediated lipid mixing. HCVpp in PBS at pH 7.2 were incubated or not with indicated concentrations of silymarin in DMSO, for 3 min at 37°C, in the presence of PC:cholesterol:R18 liposomes. Acidification to pH 5.0 was performed by adding diluted HCl to the cuvette, and R18 dequenching was assayed for 15 min at excitation and emission wavelengths of 560 and 590 nm, respectively. Maximal dequenching was obtained after addition of 0.1% final Triton X-100 to the cuvette. Black, no silymarin; blue, 10 μM; green, 20 μM and red, 80 μM silymarin, respectively. Dotted curve, lipid mixing in the presence of 1% final DMSO (highest concentration used in the assay). E, Silymarin inhibits HCV RNA production. Huh7.5.1 cells were infected at an m.o.i. of 0.01 with JFH-1 and 24 hours later, silymarin (40μM) or IFN (10 units/ml) was added to cells, and thereafter, total RNA was isolated from cells at the indicated time points. HCV RNA was quantitated by real time RT-PCR. Asterisks indicate silymarin or IFN reduction of viral loads is significantly different than untreated cells (p<0.01). F, Silymarin reduces infectious virus production into culture supernatants. Huh7.5.1 cells were infected at an m.o.i. of 0.01 with JFH-1 in the presence of 40μM silymarin or DMSO and supernatants were harvested 48 and 72 hours post-infection and titered by FFU assay on naïve Huh7.5.1 cells.
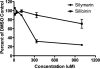
Figure 2. Silymarin inhibits JFH-1 polymerase at high dose
HCV NS5B polymerase from isolate JFH-1 (genotype 2a) was incubated with the indicated concentrations of Silymarin or Silibinin, respectively, in presence of [32P]GTP and polyC. Incorporation of radioactivity was quantified by TCA precipitation and liquid scintillation counting. Silymarin and to a lesser degree, silibinin, inhibited JFH-1 polymerase.
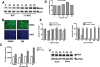
Figure 3. Silymarin does not block HCV replication in HCV replicon cell lines
Cells were treated once with the indicated doses of silymarin and incubated for 72 hours before replication was assessed. A, protein expression in subgenomic BB7 and genomic FL-NEO HCV-1b replicons following treatment with 20–80 μM of silymarin. D=DMSO. Positions of HCV NS5A and cellular Stat1 proteins are indicated. B, Intracellular NS5A protein expression in DMSO and silymarin treated FL-NEO cells. Cells were treated as described above and NS5A protein detected by immunofluorescence as described in the Materials and Methods. NS5A positive cells are depicted in green while the blue cells represent nuclei counterstained by DAPI. C, HCV RNA levels in FL-NEO and BB7 cells. D, Effect of silymarin against Luc-ubi-neo/ET cells, a Con1-based genotype 1b replicon subgenomic replicon. Values represent percent change in luciferase light units relative to DMSO control. E, Effect on HCV-1a subgenomic replicon. Left panel shows HCV and GAPDH RNA levels following Silymarin treatment, while right panel shows RNA levels following treatment with Intron-A (recombinant IFN-α). F, Effect of silymarin on subgenomic genotype 2a JFH-1-derived Huh7 replicon cell line. Cells were treated with 20 or 40μM of silymarin. Positions of HCV NS5A and cellular GAPDH proteins are indicated.
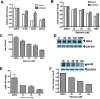
Figure 4. Silymarin inhibits microsomal triglyceride transfer protein (MTP) activity, apolipoprotein B (apoB) secretion, and infectious virus production
A, Silymarin inhibits MTP activity in Huh7.5.1 cells. Chronically infected Huh7.5.1 cells (14 days post-infection) and uninfected cells were treated with the indicated μM doses of silymarin (SM) or as a positive control, 200 μg/ml naringenin (NN), and 24 hours later MTP activity was measured as described in the Materials and Methods. B, Silymarin inhibits apoB secretion from mock and HCV-infected cells. Huh7.5.1 cells were infected or mock-infected with JFH-1 at an m.o.i. of 0.01 for 96 hours before treatment with fresh medium containing DMSO, 10μM BMS-200150 (a small molecule inhibitor of MTP), or silymarin at the indicated μM doses for 5 hours. Culture supernatants were harvested, and apoB measured by ELISA. BMS=BMS-200150. C, De novo infectious virion production into culture supernatants is blocked by silymarin. Supernatants from panel B were diluted 1:20 and infectious titers were determined by focus forming unit assay on naïve Huh7.5.1 cells. BMS=BMS-200150. D, Treatment of infected cells for 5 hours does not inhibit intracellular HCV replication. Protein lysates were harvested from cultures described in panel B and equal amounts of total protein were blotted for NS5A. The positions of HCV NS5A and loading control GAPDH are indicated. D is the DMSO control, 40, 80, and 120 are the doses of silymarin in μM, and BMS is the MTP inhibitor BMS-200150. E, Silymarin blocks apoB secretion from primary human hepatocytes. Cells were treated with the indicated concentrations of SM for 24 hours before supernatants were harvested and apoB measured by ELISA. F, Silymarin blocks apoB secretion in HepG2 cells. Cells were treated with the indicated μM concentrations of silymarin for 5 hours before supernatants were harvested and apoB measured by ELISA. Inset, Silymarin blocks intracellular apoB levels. Cell lysates from HepG2 cells treated in panel F were probed for apoB by western blot. apoB100 and loading control GAPDH are indicated with arrows. M is the media control, D is the DMSO control, and 40, 80, and 120 are the doses of silymarin in μM.
Figure 5. Silymarin inhibits total virus transmission and cell-to-cell spread
Naïve unlabelled Huh-7.5 cell targets were pre-incubated with 80μM Silymarin or equivalent volume of DMSO for 1h at 37C. The pre-treated cells were then co-cultured with CMFDA labeled H77/JFH infected Huh-7.5 producers for 48hrs. The cultures were performed in the presence of either mAb 9/27, an anti-E2 (HVR) neutralizing antibody or an anti-HIV gp120 irrelevant control, mAb 10/76B. Each treatment was performed in duplicate. The co-cultures were harvested, stained and analysed by flow cytometry and the percentage of infected cells was calculated. Cell culture media from each co-culture was tested for the presence of HCVcc particles. Released H77/JFH virus were readily detectably in media containing control antibody, no infectious particles were detected in the media containing mAb 9/27, indicating that extracellular spread of virus was not occuring and only cell-cell spread was operative. A, Silymarin (SM) reduces both total and cell:cell transmission. B, Silymarin inhibits both total and cell:cell transmission in comparable manner suggesting that it does not discriminate between the alternative routes.
Comment in
- Silibinin mode of action against hepatitis C virus: a controversy yet to be resolved.
Dahari H, Guedj J, Perelson AS. Dahari H, et al. Hepatology. 2011 Aug;54(2):749. doi: 10.1002/hep.24310. Epub 2011 Jun 27. Hepatology. 2011. PMID: 21433039 Free PMC article. No abstract available.
Similar articles
- Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase.
Ahmed-Belkacem A, Ahnou N, Barbotte L, Wychowski C, Pallier C, Brillet R, Pohl RT, Pawlotsky JM. Ahmed-Belkacem A, et al. Gastroenterology. 2010 Mar;138(3):1112-22. doi: 10.1053/j.gastro.2009.11.053. Epub 2009 Dec 4. Gastroenterology. 2010. PMID: 19962982 - Differential in vitro effects of intravenous versus oral formulations of silibinin on the HCV life cycle and inflammation.
Wagoner J, Morishima C, Graf TN, Oberlies NH, Teissier E, Pécheur EI, Tavis JE, Polyak SJ. Wagoner J, et al. PLoS One. 2011 Jan 28;6(1):e16464. doi: 10.1371/journal.pone.0016464. PLoS One. 2011. PMID: 21297992 Free PMC article. - Multiple effects of Honokiol on the life cycle of hepatitis C virus.
Lan KH, Wang YW, Lee WP, Lan KL, Tseng SH, Hung LR, Yen SH, Lin HC, Lee SD. Lan KH, et al. Liver Int. 2012 Jul;32(6):989-97. doi: 10.1111/j.1478-3231.2011.02621.x. Epub 2011 Aug 11. Liver Int. 2012. PMID: 22098176 - Silymarin for HCV infection.
Polyak SJ, Oberlies NH, Pécheur EI, Dahari H, Ferenci P, Pawlotsky JM. Polyak SJ, et al. Antivir Ther. 2013;18(2):141-7. doi: 10.3851/IMP2402. Epub 2012 Sep 25. Antivir Ther. 2013. PMID: 23011959 Free PMC article. Review.
Cited by
- Hepatitis C viral kinetics: the past, present, and future.
Chatterjee A, Smith PF, Perelson AS. Chatterjee A, et al. Clin Liver Dis. 2013 Feb;17(1):13-26. doi: 10.1016/j.cld.2012.09.003. Clin Liver Dis. 2013. PMID: 23177280 Free PMC article. Review. - Phenothiazines inhibit hepatitis C virus entry, likely by increasing the fluidity of cholesterol-rich membranes.
Chamoun-Emanuelli AM, Pecheur EI, Simeon RL, Huang D, Cremer PS, Chen Z. Chamoun-Emanuelli AM, et al. Antimicrob Agents Chemother. 2013 Jun;57(6):2571-81. doi: 10.1128/AAC.02593-12. Epub 2013 Mar 25. Antimicrob Agents Chemother. 2013. PMID: 23529728 Free PMC article. - Tissue Lipid Profiles of Rainbow Trout, Oncorhynchus mykiss, Cultivated under Environmental Variables on a Diet Supplemented with Dihydroquercetin and Arabinogalactan.
Fokina NN, Sukhovskaya IV, Kantserova NP, Lysenko LA. Fokina NN, et al. Animals (Basel). 2023 Dec 27;14(1):94. doi: 10.3390/ani14010094. Animals (Basel). 2023. PMID: 38200824 Free PMC article. - Hepatitis C virus dynamics and cellular gene expression in uPA-SCID chimeric mice with humanized livers during intravenous silibinin monotherapy.
DebRoy S, Hiraga N, Imamura M, Hayes CN, Akamatsu S, Canini L, Perelson AS, Pohl RT, Persiani S, Uprichard SL, Tateno C, Dahari H, Chayama K. DebRoy S, et al. J Viral Hepat. 2016 Sep;23(9):708-17. doi: 10.1111/jvh.12551. Epub 2016 Jun 8. J Viral Hepat. 2016. PMID: 27272497 Free PMC article. - Methanolic Extract of Rhizoma Coptidis Inhibits the Early Viral Entry Steps of Hepatitis C Virus Infection.
Hung TC, Jassey A, Lin CJ, Liu CH, Lin CC, Yen MH, Lin LT. Hung TC, et al. Viruses. 2018 Nov 27;10(12):669. doi: 10.3390/v10120669. Viruses. 2018. PMID: 30486350 Free PMC article.
References
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. - PubMed
- Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, et al. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605–612. - PubMed
- Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. - PubMed
- Rainone F. Milk thistle. Am Fam Physician. 2005;72:1285–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R21 AT002895/AT/NCCIH NIH HHS/United States
- R01 CA104286/CA/NCI NIH HHS/United States
- G0400802/MRC_/Medical Research Council/United Kingdom
- P30-DK040561/DK/NIDDK NIH HHS/United States
- CA104286/CA/NCI NIH HHS/United States
- AI-25488/AI/NIAID NIH HHS/United States
- N01 AI040034/AI/NIAID NIH HHS/United States
- AT002895/AT/NCCIH NIH HHS/United States
- G0801976/MRC_/Medical Research Council/United Kingdom
- R21 CA125321/CA/NCI NIH HHS/United States
- R01 AI050798/AI/NIAID NIH HHS/United States
- AI40034-14/AI/NIAID NIH HHS/United States
- K01-DK080241/DK/NIDDK NIH HHS/United States
- K01 DK080241/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- AI50798/AI/NIAID NIH HHS/United States
- N01 AI025488/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous