Climate change and freshwater ecosystems: impacts across multiple levels of organization - PubMed (original) (raw)
Review
Climate change and freshwater ecosystems: impacts across multiple levels of organization
Guy Woodward et al. Philos Trans R Soc Lond B Biol Sci. 2010.
Abstract
Fresh waters are particularly vulnerable to climate change because (i) many species within these fragmented habitats have limited abilities to disperse as the environment changes; (ii) water temperature and availability are climate-dependent; and (iii) many systems are already exposed to numerous anthropogenic stressors. Most climate change studies to date have focused on individuals or species populations, rather than the higher levels of organization (i.e. communities, food webs, ecosystems). We propose that an understanding of the connections between these different levels, which are all ultimately based on individuals, can help to develop a more coherent theoretical framework based on metabolic scaling, foraging theory and ecological stoichiometry, to predict the ecological consequences of climate change. For instance, individual basal metabolic rate scales with body size (which also constrains food web structure and dynamics) and temperature (which determines many ecosystem processes and key aspects of foraging behaviour). In addition, increasing atmospheric CO(2) is predicted to alter molar CNP ratios of detrital inputs, which could lead to profound shifts in the stoichiometry of elemental fluxes between consumers and resources at the base of the food web. The different components of climate change (e.g. temperature, hydrology and atmospheric composition) not only affect multiple levels of biological organization, but they may also interact with the many other stressors to which fresh waters are exposed, and future research needs to address these potentially important synergies.
Figures
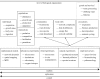
Figure 1.
Schematic of studies into climate change impacts in fresh waters arranged along gradients of control, replication and realism, highlighting the links between different levels of organization investigated and the approaches used.
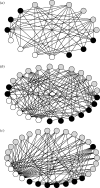
Figure 2.
Stream benthic food webs along a thermal gradient in the Estaragne Basin, French Pyrénées. Open circles denote basal resources; grey denotes primary consumers; black denotes predators. (a) 2370 m altitude, maximum water temperature (T_max) = 4.5°C, no. species (S) = 16, secondary production (2_P) = 4.9 g m−2 y−1. (b) 2150 m altitude, _T_max = 8.5°C, S = 25, 2_P_=6.55 g m−2 y−1. (c) 1850 m altitude, T_max = 13°C, S = 30, 2_P = 7.6 g m−2 y−1. Figures redrawn from Lavandier & Décamps (1983).
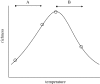
Figure 3.
Hypothetical unimodal relationship between local species richness and temperature. In relatively equitable environments, where most species are close to their thermal optima, we might expect richness to decline with warming (trajectory B). However, this scenario is unlikely to be ubiquitous: in systems close to the physiological limits of existence for most organisms (e.g. in polar regions: trajectory A), increases in temperature may lead to rises in local richness following invasion by less cold-tolerant species. This scenario, however, implies a loss of global diversity as specialist cold stenotherms are replaced by the more diverse eurytherms.
Figure 4.
Potential effects of elevated temperature and altered nutrient quality of resources on metabolic (a,b,c,d), foraging (e,f,g,h) and stoichiometric constraints (i,j,k,l) over multiple levels of biological organization (individual, population, community and ecosystem). The relationships with temperature relate primarily to the ectotherms that dominate freshwater communities. All axes are log–log scales. (a) Per capita BMR increases with body mass (indicated by the solid line) with the scaling exponent, β = ¾ (Peters 1983). Metabolic demands will rise with warming (Brown et al. 2004; Yvon-Durocher et al. 2010; dashed line). (b) Mass-dependence of population abundance (solid line), where a common exponent of β =−¾ is predicted (Brown et al. 2004). As individual metabolic rates rise, warming is likely to result in a decrease in total biomass, given steady-state conditions (Brown et al. 2004). (c) Body-mass–abundance relationships across trophic levels (solid line), where a scaling exponent β =−1 is predicted (Brown et al. 2004; Woodward et al. 2005). In addition to changes in total biomass (dashed line), warming should induce transient changes in body size spectra as large, rare species high in the food web are lost first (e.g. Petchey et al. 1999; dashed arrow). In contrast, in very cold systems under physiological stress, warming could stimulate productivity, allowing food chains to lengthen and the size spectrum to broaden (Lavandier & Décamps 1983; Friberg et al. 2009; Milner et al. 2009; solid arrow). (d) Relationship between rates of resource turnover and average consumer body mass (solid line). Resources flux faster through assemblages of small individuals: the exponent of this relationship follows that of mass-specific metabolic rates, β = −¼ (Brown et al. 2004). Thus, warming will elevate process rates and changes in community size structure will also have consequences at the ecosystem level (bi-directional arrow). (e) Per capita foraging capacity (solid line) increases with body mass with an exponent β < 1 (Persson et al. 2000; Bystrom & Andersson 2005). Warming will elevate individual foraging rates (dashed line) (Woodward & Hildrew 2002). (f) Critical resource density (CRD), whereby energy intake balances metabolic demands, increases with increasing body size (solid line). Within a population, this could give smaller individuals a competitive advantage (Persson 1985) as warming should increase the CRD required by consumers (dashed line) and may further favour small individuals in the population (solid arrow). (g) Predation matrix describing a food web with resources (rows) and consumers (columns) (e.g. Beckerman et al. 2006), ordered in body size bins. Circles denote realized feeding links, and those below the 1 : 1 line indicate consumers feeding on resources smaller than themselves (Petchey et al. 2008). Here, the largest consumer displays adaptive feeding on successively smaller resources (open circles) as a result of size-dependent foraging and higher metabolic offset with warming (solid arrow). (h) Mass-specific resource use decreases with body mass (solid line) and is likely to be displaced by warming (dashed line) in a comparable manner to the relationship predicted by MS (d). Similarly, associated changes in community size structure have consequences at the ecosystem level (bi-directional arrow). (i) Per capita rates of exploitation of a given resource may (a) increase with increasing consumer–resource stoichiometric imbalances (e.g. C : N or C : P ratios) if individuals feed in a compensatory manner (dotted line), or (b) are temporally impaired if consumers switch to alternative resources (dashed line) (e.g. Sterner & Elser 2002). (j) Increased inefficiencies resulting from consumer–resource stoichiometric imbalances should slow population biomass growth rates (e.g. Tuchman et al. 2002; dotted line). These effects might be mitigated to some extent if stoichiometric demands can be met by switching to alternative resources (e.g. herbivory versus detritivory, after Ledger & Hildrew 2001; dashed line). (k) Body mass–abundance relationships (solid line) comparable to those predicted by MS (c). The scaling exponent could increase if assimilation efficiency across trophic levels is reduced via lower nutritional quality of resources and suppressed biomass production (e.g. Tuchman et al. 2002; dashed line). (l) Ecosystem rates of resource turnover increase (at least transiently) with increasing stoichiometric imbalances due to the compensatory feeding performances of individuals (dotted line), or decrease due to resource switching and/or reduced biomass production (dashed line).
Similar articles
- Consumer-driven nutrient dynamics in freshwater ecosystems: from individuals to ecosystems.
Atkinson CL, Capps KA, Rugenski AT, Vanni MJ. Atkinson CL, et al. Biol Rev Camb Philos Soc. 2017 Nov;92(4):2003-2023. doi: 10.1111/brv.12318. Epub 2016 Dec 23. Biol Rev Camb Philos Soc. 2017. PMID: 28008706 Review. - Implications of climate change for the fishes of the British Isles.
Graham CT, Harrod C. Graham CT, et al. J Fish Biol. 2009 Apr;74(6):1143-205. doi: 10.1111/j.1095-8649.2009.02180.x. J Fish Biol. 2009. PMID: 20735625 Review. - Multiple stressors, nonlinear effects and the implications of climate change impacts on marine coastal ecosystems.
Hewitt JE, Ellis JI, Thrush SF. Hewitt JE, et al. Glob Chang Biol. 2016 Aug;22(8):2665-75. doi: 10.1111/gcb.13176. Epub 2016 May 12. Glob Chang Biol. 2016. PMID: 26648483 - Genetic and plastic rewiring of food webs under climate change.
Barbour MA, Gibert JP. Barbour MA, et al. J Anim Ecol. 2021 Aug;90(8):1814-1830. doi: 10.1111/1365-2656.13541. Epub 2021 Jun 22. J Anim Ecol. 2021. PMID: 34028791 Free PMC article. Review. - Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems.
Griffith AW, Gobler CJ. Griffith AW, et al. Harmful Algae. 2020 Jan;91:101590. doi: 10.1016/j.hal.2019.03.008. Epub 2019 May 21. Harmful Algae. 2020. PMID: 32057338 Review.
Cited by
- Strong and recurring seasonality revealed within stream diatom assemblages.
Snell MA, Barker PA, Surridge BWJ, Benskin CMH, Barber N, Reaney SM, Tych W, Mindham D, Large ARG, Burke S, Haygarth PM. Snell MA, et al. Sci Rep. 2019 Mar 1;9(1):3313. doi: 10.1038/s41598-018-37831-w. Sci Rep. 2019. PMID: 30824739 Free PMC article. - Thermal carrying capacity for a thermally-sensitive species at the warmest edge of its range.
Ayllón D, Nicola GG, Elvira B, Parra I, Almodóvar A. Ayllón D, et al. PLoS One. 2013 Nov 25;8(11):e81354. doi: 10.1371/journal.pone.0081354. eCollection 2013. PLoS One. 2013. PMID: 24282584 Free PMC article. - Novel environments induce variability in fitness-related traits.
Balph AW, Krist AC. Balph AW, et al. Ecol Evol. 2023 Jun 6;13(6):e10165. doi: 10.1002/ece3.10165. eCollection 2023 Jun. Ecol Evol. 2023. PMID: 37287851 Free PMC article. - Long-Term Acclimation to Different Thermal Regimes Affects Molecular Responses to Heat Stress in a Freshwater Clam Corbicula Fluminea.
Falfushynska HI, Phan T, Sokolova IM. Falfushynska HI, et al. Sci Rep. 2016 Dec 20;6:39476. doi: 10.1038/srep39476. Sci Rep. 2016. PMID: 27995990 Free PMC article. - Two sides of a coin: Effects of climate change on the native and non-native distribution of Colossoma macropomum in South America.
Lopes TM, Bailly D, Almeida BA, Santos NCL, Gimenez BCG, Landgraf GO, Sales PCL, Lima-Ribeiro MS, Cassemiro FAS, Rangel TF, Diniz-Filho JAF, Agostinho AA, Gomes LC. Lopes TM, et al. PLoS One. 2017 Jun 27;12(6):e0179684. doi: 10.1371/journal.pone.0179684. eCollection 2017. PLoS One. 2017. PMID: 28654663 Free PMC article.
References
- Acuna V., Wolf A., Uehlinger U., Tockner K.2008Temperature dependence of stream benthic respiration in an Alpine river network under global warming. Freshw. Biol. 53, 2076–2088 (doi:10.1111/j.1365-2427.2008.02028.x) - DOI
- Allen A. P., Gillooly J. F.2009Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 12, 369–384 (doi:10.1111/j.1461-0248.2009.01302.x) - DOI - PubMed
- Babaluk J. A., Reist J. A., Johnson J. D., Johnson L.2000First records of sockeye (Oncorhynchus nerka) and pink salmon (O. gorbuscha) from Banks Island and other records of Pacific salmon in Northwest Territories, Canada. Arctic 53, 161–162
- Barlocher F., Seena S., Wilson K. P., Williams D. D.2008Raised water temperature lowers diversity of hyporheic aquatic hyphomycetes. Freshw. Biol 53, 368–379
- Barnett T. P., Adam J. C., Lettenmaier D. P.2005Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438, 303–309 (doi:10.1038/nature04141) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical