Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation - PubMed (original) (raw)
Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation
Zdenek Berger et al. Biochemistry. 2010.
Abstract
Autosomal dominant mutations in leucine rich repeat kinase 2 (LRRK2) are the most common genetic cause of Parkinson's disease (PD). Despite the presence of multiple domains, the kinase activity of LRRK2 is thought to represent the primary function of the protein. Alterations in LRRK2 kinase activity are thought to underlie the pathogenesis of its PD-linked mutations; however, many questions regarding basic aspects of LRRK2 function remain unclear, including the cellular mechanisms of LRRK2 regulation and the importance of its unique distribution within the cell. Here, we demonstrate for the first time that the subcellular localization of wild-type LRRK2 is associated with changes in four distinct biochemical properties likely crucial for LRRK2 function. Our data demonstrate for the first time that the wild-type LRRK2 dimer possesses greater kinase activity than its more abundant monomeric counterpart. Importantly, we show that this activated form of LRRK2 is substantially enriched at the membrane of cells expressing endogenous or exogenous LRRK2, and that the membrane-associated fraction of LRRK2 likewise possesses greater kinase activity than cytosolic LRRK2. In addition, membrane-associated LRRK2 binds GTP more efficiently than cytosolic LRRK2 but demonstrates a lower degree of phosphorylation. Our observations suggest that multiple events, including altered protein-protein interactions and post-translational modifications, contribute to the regulation of LRRK2 function, through modulation of membrane association and complex assembly. These findings may have implications for the sites of LRRK2 function within the cell, the identification and localization of bona fide LRRK2 substrates, and efforts to design small molecule inhibitors of LRRK2.
Figures
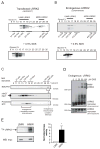
Figure 1. Analysis of HMW and LMW pools of LRRK2 and their associated kinase activity from whole-cell lysates
(A) Transiently transfected myc-LRRK2 from whole cell lysates is present in two distinct pools, as assessed by glycerol velocity gradients - low molecular weight (LMW) and high molecular weight (HMW). Glycerol gradients were calibrated using commercially available proteins of known molecular weight. Western blots from glycerol gradient fractions show that LMW LRRK2 migrates at ~230 kDa, likely representing a monomer, while HMW LRRK2 is found at approximately double the molecular weight (~440 kDa), consistent with a dimer. Addition of 0.5% SDS prior to glycerol gradients collapses HMW LRRK2. (B) Endogenous LRRK2 from human lymphoblasts is present in two distinct pools (LMW, HMW), similar to transfected LRRK2. Western blots from glycerol gradient fractions were probed with anti-myc (A) and anti-LRRK2 antibodies (B). (C) Endogenous γ-secretase complex, a protease of 230–250 kDa, is present in the same glycerol gradient fractions (15–19%) as LMW LRRK2. Cell lysates of HEK293FT cells transfected with myc-LRRK2 were separated using glycerol velocity gradients and analyzed using Western blots for endogenous components of the γ-secretase complex. Immature nicastrin (iNCT) undergoes further glycosylation to generate mature nicastrin (mNCT); only mNCT is part of the fully assembled γ-secretase complex. The N-terminal fragment of presenilin (NTF) is also selectively present in the fully assembled γ-secretase complex. Mitochondrial complex I (980kDa) is found in 35% glycerol, as evidenced by the presence of one its subunits - endogenous NDUFA9. (D) Chemical crosslinking of live cells leads to the formation of HMW complexes of endogenous LRRK2. Live lymphoblasts expressing endogenous LRRK2 were crosslinked with increasing concentrations of DSS, leading to a dose-dependent formation of SDS-stable HMW LRRK2 complexes. (E) HMW LRRK2 is more active than LMW. Wild-type myc-LRRK2 from transfected HEK293FT cells was separated into LMW and HMW pools using glycerol gradients, IPed using myc resin and subjected to an autophosphorylation assay of kinase activity. An autoradiograph of an SDS-PAGE gel separating the LRRK2 kinase reaction products shows increased incorporation of radioactive 32P into HMW LRRK2 compared to LMW LRRK2. Similar levels of LRRK2 were present in both reactions, as analyzed by Western blot. Relative kinase activity of HMW LRRK2 is 8.4-fold greater than that of LMW LRRK2 (Mean ± SEM_, p_ = 0.03, n = 6, unpaired t-test). * p < 0.05.
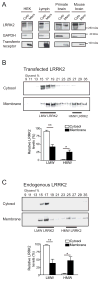
Figure 2. The high molecular weight LRRK2 complex is enriched at the membrane
(A) A higher proportion of LRRK2 is localized in cytosol than at the membrane. HEK293FT cells (HEK), lymphoblasts (Lymph), primate or mouse brains were homogenized and fractionated into cytosol (Cyto) and membrane (Mem) fractions. Both fractions were volume-normalized and analyzed by SDS-PAGE/Western blot. GAPDH and transferrin receptor were used as controls for proteins in the cytosol and membrane fractions, respectively. (B) Glycerol gradients of cytosol and membrane extracts from HEK293FT cells transfected with myc-LRRK2 reveal a 20-fold enrichment of HMW LRRK2 at the membrane compared to the cytosol (p = 0.02). A higher proportion of LMW LRRK2 is found in the cytosol than in the membrane compartment (Mean ± SEM, p = 0.004, n = 4, Student’s t-test). * p < 0.05, ** p ≤ 0.01. (C) Analysis of endogenous LRRK2 from human lymphoblasts reveals an enrichment of its HMW complex at the membrane, similar to that observed in (B) with transfected LRRK2. The levels of HMW LRRK2 are 15-fold higher in membrane than cytosolic fractions (Mean ± SEM_, p_ = 0.01, n = 4, Student’s t-test). * p < 0.05, ** p ≤ 0.01.
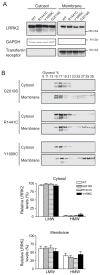
Figure 3. PD-linked mutants do not influence the subcellular localization or oligomerization state of LRRK2
(A) PD-linked LRRK2 mutants and wild-type LRRK2 show similar distribution across the cytosol and membrane compartments when expressed in HEK293FT cells. (B) Glycerol gradients of cytosol and membrane fractions from HEK293FT cells transfected with PD-linked LRRK2 mutants. The distribution of LMW and HMW LRRK2 in either cytosol or membrane fractions is not significantly changed by the introduction of PD-linked LRRK2 mutations (G2019S, R1441C, Y1699C) (Mean ± SEM, n = 3, Student’s t-test, all p > 0.05).
Figure 4. Heterologous co-immunoprecipitation confirms an enrichment of the LRRK2 dimer at the membrane
(A) To optimize the heterologous co-immunoprecipitation (co-IP) system, the amount of myc-LRRK2 DNA was titrated to match the expression levels of GFP-LRRK2. Transfection with 0.5 μg of myc-LRRK2 led to similar levels of LRRK2 protein as 5 μg of GFP-LRRK2. (B) A schematic depicting possible LRRK2 dimers after transfection with GFP-LRRK2 and myc-LRRK2 plasmids. Co-IP of heterologously tagged constructs allows quantification of GFP-LRRK2/myc-LRRK2 heterodimer, which at equal levels of both proteins will likely represent 50% of total dimer. (C) Cytosol and membrane fractions from cells co-expressing GFP-LRRK2 and myc-LRRK2 (lanes 1–2) or GFP-LRRK2 and an empty vector (lanes 3–4) were used for IP using a high affinity myc resin. GFP-LRRK2 is co-IPed only in the presence of myc-LRRK2 (lanes 5–9), confirming specificity of IP. Higher levels of GFP-LRRK2 are pulled down from membrane extracts (lane 7), despite lower levels of myc-LRRK2 in the same IP, suggesting an enrichment of LRRK2 dimer at the membrane. Identical results from two independent samples (C1, C2) using the same total cytosolic fraction (CT) illustrate the low variability of this assay. (D), Levels of LRRK2 heterodimers are 5.7-fold higher in membrane extracts (Mean ± SEM, p = 0.01, n = 3, unpaired t-test). The relative dimer levels were quantified by measuring the band intensity of co-IPed GFP-LRRK2 divided by the intensity of IPed myc-LRRK2. The value for cytosol was arbitrarily set as 1. ** p ≤ 0.01.
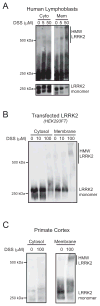
Figure 5. Membrane bound LRRK2 can be crosslinked more efficiently into HMW complexes than cytosolic LRRK2
Crosslinking with DSS of endogenous or transfected LRRK2 leads to formation of HMW complexes in membrane fractions (Mem), but not in cytosol (Cyto). Extracts from human lymphoblasts (A), HEK293FT cells transfected with LRRK2 (B), and non-human primate cortex (C) were analyzed.
Figure 6. Analysis of LRRK2 by size exclusion chromatography and Blue-Native PAGE
(A) Cytosolic LRRK2 and LMW LRRK2 (both monomer) elute similarly from a Superdex 200 column, at ~ 1.3MDa, while HMW LRRK2 (LRRK2 dimer) elutes at ~ 500kDa. (B) LMW LRRK2 from the glycerol gradient separation subsequently migrates on BN-PAGE as a LRRK2 monomer, whereas migration of HMW LRRK2 corresponds to a dimer. Two commercially available molecular weight standards were used on the Blue-Native PAGE to estimate LRRK2 molecular weight (see Figure S4).
Figure 7. Membrane-associated LRRK2 is biochemically distinct from cytosolic LRRK2
(A) Membrane-associated LRRK2 exhibits greater in vitro kinase activity than cytosolic LRRK2. Cytosol and membrane fractions from HEK293FT cells transfected with wild-type myc-LRRK2 or from untransfected cells (Untrans) were used for myc IP and subsequent LRRK2 autophosphorylation assay. Membrane and cytosolic fractions were normalized to achieve similar levels of LRRK2 in the kinase reaction, shown by Western blot. Autoradiograph of an SDS-PAGE gel separating the LRRK2 autophosphorylation reaction products show increased incorporation of radioactive 32P into LRRK2 from membrane extracts. LRRK2 from the cytosolic fraction migrates on this gel as a doublet; both bands correspond to full-length LRRK2 and were included in the analysis. Relative LRRK2 kinase activity is 3.1-fold greater from membrane fraction than from cytosol (Mean ± SEM_, p_ = 0.04, n = 3, unpaired t-test). * p < 0.05. (B) Membrane-associated LRRK2 from human lymphoblasts binds GTP more efficiently than cytosolic LRRK2. The levels of LRRK2 were normalized in the input and equal volumes of inputs were used for each analysis, with three independent experiments demonstrating similar results. (C) Membrane-associated LRRK2 extracted from primate brain binds GTP more efficiently than cytosolic LRRK2. LRRK2 was first eluted with GTP from the resin and subsequently the GTP binding resin was boiled in Laemmli buffer. Samples used for the assay were protein and volume normalized, a representative experiment (n =3) is shown. (D) Membrane-associated LRRK2 is phosphorylated to a lesser extent than cytosolic LRRK2. Cytosolic and membrane fractions from untransfected cells (Untrans) or cells transfected with wild-type myc-LRRK2 (WT LRRK2) were IPed with myc resin, and analyzed for phosphorylation using Pro-Q® Diamond Phosphoprotein gel stain (Phospho-LRRK2). The relative levels of LRRK2 phosphorylation were quantified by normalizing the intensity of phospho-LRRK2 (phospho-protein stain) to the levels of total LRRK2 protein by SYPRO® Ruby stain (total LRRK2 S-R). Relative LRRK2 levels were further confirmed by analyzing a small aliquot of each fraction by Western blot (total LRRK2 WB). (Mean ± SEM_, p_ = 0.01, n = 3, unpaired t-test). ** p ≤ 0.01
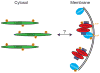
Figure 8. Schematic representation of proposed model of LRRK2 dimer assembly and kinase regulation
Using both endogenous and exogenous LRRK2, we have observed that membrane-associated LRRK2 is substantially enriched for LRRK2 dimer, whereas cytosolic LRRK2 is present mostly as a monomer. Membrane-associated LRRK2 possesses greater kinase activity, an increased propensity to bind GTP, and is relatively dephosphorylated, compared to cytosolic LRRK2. We propose a model, where LRRK2 exists mostly as a monomer in the cytosol that can translocate to membrane where it dimerizes, becomes more active and subsequently phosphorylates its substrates. The similarities of this model to the established regulation of other kinases (and GTPases) suggest that membrane translocation and dimerization may be reversible and tightly controlled.
Similar articles
- Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization.
Sen S, Webber PJ, West AB. Sen S, et al. J Biol Chem. 2009 Dec 25;284(52):36346-36356. doi: 10.1074/jbc.M109.025437. Epub 2009 Oct 13. J Biol Chem. 2009. PMID: 19826009 Free PMC article. - Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants.
Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Li X, et al. J Neurochem. 2007 Oct;103(1):238-47. doi: 10.1111/j.1471-4159.2007.04743.x. Epub 2007 Jul 10. J Neurochem. 2007. PMID: 17623048 Free PMC article. - The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity.
Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. Guo L, et al. Exp Cell Res. 2007 Oct 1;313(16):3658-70. doi: 10.1016/j.yexcr.2007.07.007. Epub 2007 Jul 19. Exp Cell Res. 2007. PMID: 17706965 Free PMC article. - New biochemical approaches towards understanding the Parkinson's disease-associated kinase, LRRK2.
Liou GY, Gallo KA. Liou GY, et al. Biochem J. 2009 Oct 23;424(1):e1-3. doi: 10.1042/BJ20091540. Biochem J. 2009. PMID: 19839940 Review. - Leucine-rich repeat kinase 2 (LRRK2) cellular biology: a review of recent advances in identifying physiological substrates and cellular functions.
Drolet RE, Sanders JM, Kern JT. Drolet RE, et al. J Neurogenet. 2011 Dec;25(4):140-51. doi: 10.3109/01677063.2011.627072. Epub 2011 Nov 11. J Neurogenet. 2011. PMID: 22077787 Review.
Cited by
- The neurobiology of LRRK2 and its role in the pathogenesis of Parkinson's disease.
Rideout HJ, Stefanis L. Rideout HJ, et al. Neurochem Res. 2014;39(3):576-92. doi: 10.1007/s11064-013-1073-5. Epub 2013 Jun 1. Neurochem Res. 2014. PMID: 23729298 Review. - GTP-binding protein-like domain of AGAP1 is protein binding site that allosterically regulates ArfGAP protein catalytic activity.
Luo R, Akpan IO, Hayashi R, Sramko M, Barr V, Shiba Y, Randazzo PA. Luo R, et al. J Biol Chem. 2012 May 18;287(21):17176-17185. doi: 10.1074/jbc.M111.334458. Epub 2012 Mar 27. J Biol Chem. 2012. PMID: 22453919 Free PMC article. - Is Glial Dysfunction the Key Pathogenesis of _LRRK2_-Linked Parkinson's Disease?
Iseki T, Imai Y, Hattori N. Iseki T, et al. Biomolecules. 2023 Jan 15;13(1):178. doi: 10.3390/biom13010178. Biomolecules. 2023. PMID: 36671564 Free PMC article. Review. - Proteostasis and movement disorders: Parkinson's disease and amyotrophic lateral sclerosis.
Bosco DA, LaVoie MJ, Petsko GA, Ringe D. Bosco DA, et al. Cold Spring Harb Perspect Biol. 2011 Oct 1;3(10):a007500. doi: 10.1101/cshperspect.a007500. Cold Spring Harb Perspect Biol. 2011. PMID: 21844169 Free PMC article. Review. - Structural biology of the LRRK2 GTPase and kinase domains: implications for regulation.
Gilsbach BK, Kortholt A. Gilsbach BK, et al. Front Mol Neurosci. 2014 May 5;7:32. doi: 10.3389/fnmol.2014.00032. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24847205 Free PMC article. Review.
References
- Thomas B, Beal MF. Parkinson’s disease. Human molecular genetics. 2007;16(Spec No 2):R183–194. - PubMed
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. - PubMed
- Cookson MR, Xiromerisiou G, Singleton A. How genetics research in Parkinson’s disease is enhancing understanding of the common idiopathic forms of the disease. Current opinion in neurology. 2005;18:706–711. - PubMed
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 NS038375-02/NS/NINDS NIH HHS/United States
- P50 NS038375/NS/NINDS NIH HHS/United States
- AG023094/AG/NIA NIH HHS/United States
- NS038375/NS/NINDS NIH HHS/United States
- K01 AG023094-01A2/AG/NIA NIH HHS/United States
- K01 AG023094/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases