Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo - PubMed (original) (raw)
. 2010 Jul 7;29(13):2147-60.
doi: 10.1038/emboj.2010.106. Epub 2010 Jun 1.
Gwenael Badis, Michael F Berger, Teemu Kivioja, Kimmo Palin, Martin Enge, Martin Bonke, Arttu Jolma, Markku Varjosalo, Andrew R Gehrke, Jian Yan, Shaheynoor Talukder, Mikko Turunen, Mikko Taipale, Hendrik G Stunnenberg, Esko Ukkonen, Timothy R Hughes, Martha L Bulyk, Jussi Taipale
Affiliations
- PMID: 20517297
- PMCID: PMC2905244
- DOI: 10.1038/emboj.2010.106
Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo
Gong-Hong Wei et al. EMBO J. 2010.
Abstract
Members of the large ETS family of transcription factors (TFs) have highly similar DNA-binding domains (DBDs)-yet they have diverse functions and activities in physiology and oncogenesis. Some differences in DNA-binding preferences within this family have been described, but they have not been analysed systematically, and their contributions to targeting remain largely uncharacterized. We report here the DNA-binding profiles for all human and mouse ETS factors, which we generated using two different methods: a high-throughput microwell-based TF DNA-binding specificity assay, and protein-binding microarrays (PBMs). Both approaches reveal that the ETS-binding profiles cluster into four distinct classes, and that all ETS factors linked to cancer, ERG, ETV1, ETV4 and FLI1, fall into just one of these classes. We identify amino-acid residues that are critical for the differences in specificity between all the classes, and confirm the specificities in vivo using chromatin immunoprecipitation followed by sequencing (ChIP-seq) for a member of each class. The results indicate that even relatively small differences in in vitro binding specificity of a TF contribute to site selectivity in vivo.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
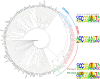
Structural organizations and binding specificities of mammalian ETS transcription factors. (Left) Schematic representation of the domain structures of the respective full-length proteins. ETS domain is in blue, pointed domain is in green, Proline-rich domain is in grey, and the Nuc_orp_HMR_rcpt and A/T hook domains are in dark yellow and black, respectively. HUGO gene names are from ENSEMBL and protein domains are from Pfam. The second and third columns, respectively, show human and mouse ETS-binding profiles determined using microwell-based transcription factor-DNA-binding assays. The right column shows mouse ETS-binding profiles determined using protein-binding microarrays. The logos are drawn using enoLOGOS (Workman et al, 2005), and the height of a letter at a particular position is directly proportional to the effect of that nucleotide on the binding affinity. Coordinates for the bases are also indicated above each column (see also Supplementary Figures S1 and S9; Supplementary Tables S1, S2 and S6; Supplementary data file S1).
Figure 2
ETS-binding specificity. Clustering analysis of binding profiles of human (h) and mouse (m) ETS transcription factors (microwell method) and publicly available non-ETS-family transcription factor matrices from Jaspar2 (Bryne et al, 2008;
). The four different classes of ETS factors are indicated by colour: class I, red; class II, blue; class III, green; class IV, brown. Coloured dots indicate the main branches defining the classes. ETS matrices indicated as ‘class' are the representative matrices for the different ETS classes identified using affinity propagation clustering (see Materials and methods for details). Representative logos, drawn using enoLOGOS (Workman et al, 2005), are also shown. Bases displaying the most prominent changes are boxed (see also Supplementary Figures S2 and S6; Supplementary data file S1).
Figure 3
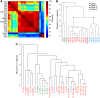
Identification of four ETS classes is independent of clustering and binding model derivation methods used. (A) Heat-map correlation analysis of the protein-binding microarray-derived ETS-binding models. The same four ETS classes are detected when protein-binding microarray results are clustered using the top 100 TF-binding sites for each ETS-family member (analysis as in Berger et al, 2008). In all, 20 known and 2 predicted mouse ETS-family members are included in the analysis. (B) Kullback–Leibler divergence-based clustering analysis of mouse ETS-binding profiles derived from protein-binding microarrays. Note that this analysis also reveals the same four different classes of ETS factors, which are indicated in the same colours as in Figures 1 and 2. Coloured dots indicate the main branches defining the classes. (C) Clustering of existing ETS family binding profiles from JASPAR2 (JA), TRANSFAC (TR) professional and literature (Nye et al, 1992; Treisman et al, 1992; Woods et al, 1992; Dalton and Treisman, 1992; Virbasius et al, 1993; Ray-Gallet et al, 1995; Shore and Sharrocks, 1995; Matys et al, 2006; Choi and Sinha, 2006; Bryne et al, 2008). Note that the four separate ETS classes are not identified using the earlier data (see also Supplementary Figures S3 and S6).
Figure 4
Molecular basis of ETS-class specificity. (A) ETS-domain secondary structure indicating key amino acids contacting nucleotide bases (black lines), DNA-backbone (brown lines) and water (blue line) based on the published crystal structures of ETS-domain DNA complexes (Kodandapani et al, 1996; Batchelor et al, 1998; Mo et al, 1998, 2000; Garvie et al, 2001; Pufall et al, 2005; Wang et al, 2005; Lamber et al, 2008; Agarkar et al, 2010). Amino-acid residues contacting DNA are numbered from 1 to 15. Two invariant arginines that bind to the core GGA sequence are in black typeface. Bases contributing to DNA-binding specificity are numbered from −3 to +7. (B) (Left) Sequence logos showing amino-acid conservation in DNA-contacting regions of the different ETS classes. Amino acids that are specific for a given class are indicated by colours, and the two invariant arginines are in black. (Right) Representative PWMs for the ETS classes. Bases that distinguish each class from the others are boxed, and residues that contact bases, water or DNA backbone are indicated in black, blue or yellow lines, respectively. (C) Identification of amino-acid residues that are required for class IV DNA-binding specificity. Mutating key DNA-contact residues in ETS class IV factor SPDEF (top) to the corresponding residues in ETS class IIa (bottom left) results in a change in DNA-binding specificity of SPDEF towards class IIa (bottom right; data from microwell assay). (D) Identification of amino-acid residues that can confer class I, IIb or III DNA-binding specificity to a class IIa ETS factor. Indicated residues in class IIa ETS DNA-binding domain from mouse ELF4 were mutated to the corresponding residues in the other ETS classes. The resulting DNA-binding profile from microwell assay is shown on the right. Bases displaying the most prominent changes are boxed. Note that one or two amino-acid mutations can move the specificity of ELF4 towards the other ETS classes. Residues whose mutation to the corresponding residues in class III had no effect on specificity are indicated in yellow.
Figure 5
Crystal structures displaying residues that are critical for ETS-class specificity. (A) Tyrosine 410 (red) in strand-4 of ETS1 (class I) contacting DNA backbone between positions −3 and −2 (base G at −3 indicated in blue). This residue is responsible for exclusion of C at position −3. (B) Glutamine 228 in helix-3 of SPI1 (class III) contacting water molecules (red spheres indicated by asterisks) near DNA positions −1 (blue) and −2 (cyan). This residue is responsible for preference of G at −2, and weaker binding to C at −1. (C) Asparagine 236 in helix-3 of SPI1 (class III) contacting an adenine at +4 position. This residue is responsible for stronger preference for A at +4 and higher-affinity binding to sites containing a C at +5. Top parts of each panel display sequence logos for the classes and the DNA sequences used in the crystallization (Kodandapani et al, 1996; Garvie et al, 2001), with the class-specific amino-acid residues and bases indicated by colouring. Contacts between amino acids and DNA backbone, water or bases are indicated using brown, red or black lines, respectively. Images were generated using PolyView-3D (
http://polyview.cchmc.org/polyview3d.html
).
Figure 6
ChIP-seq analysis of the different ETS classes. (A) Relative enrichment of ChIP-seq peaks with respect to transcription start sites. Note that ELF1 peaks are strongly enriched in promoters, whereas the other non-fusion ETS-family factors ERG, SPDEF and SPI1 display smaller promoter enrichment. The ETS factors fused to the EWS protein (EWS/FLI1 and EWS/ERG) display the smallest enrichment at promoters. (B) Enrichment of FLI1 ChIP-seq peaks near transcription start sites of genes that are downregulated in response to FLI1 siRNAs. (C) Analysis of specific enrichment of ETS class PWM matches in ChIP-seq peak sequences. Note that sequences immunoprecipitated using antibodies against a member of a given ETS DNA-binding class are enriched in matches to the PWM representing the same class. (D) MEME analysis of enrichment of sequence motifs in ChIP-seq peaks from experiments analysing members of all ETS classes (ERG, ELF1, SPI1 and SPDEF). Note that peaks in ChIP-seq experiments are enriched in motifs (in vivo, bottom) that are similar to those obtained using microwell assay (in vitro, top). Note also that the in vivo analysis confirms the differences in specificity of the ETS classes at the class-specific positions (boxes; see Figure 2) identified in vitro. The differences observed between the in vitro and in vivo profiles mainly affect the −1 and +5 positions of ERG and ELF1, respectively. These differences could be at least in part due to the presence of a high number of GGAA repeat containing ETS sites in the human genome, as −1 and +5 positions in sites derived from such repeats are enriched in A and G, respectively. These GGAA repeat-derived sequences can be functionally important (Gangwal et al, 2008). Colour code: class I, red; class II, blue; class III, green; class IV, brown (see also Supplementary Figures S8 and S10; Supplementary Tables S3–S6; Supplementary data files S2, S3, S4, S5, S6, S7, S8).
Figure 7
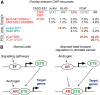
Analysis of ChIP-seq data. (A) Overlap between ChIP-seq peaks. All overlaps are significantly enriched compared with random expectation (P<0.001), with exception of ELF1 versus AR (in grey, significance _P_=0.0013). _P_-values for each overlap are listed in Supplementary Table S4. Note that the highest observed overlap (red bold typeface) in all experiments is between AR and ERG. Other overlaps over 10% are coloured in brown. (B) Model of aberrant feed-forward regulation of common androgen receptor and ETS targets in prostate cancer. In normal cells (left), activation of AR and ETS-family TFs requires separate signals. In prostate cancer (right), a strong AR-regulated element is fused to ERG, ETV1 or ETV4 (indicated by ETS), resulting in an aberrant feed-forward loop (arrows) (see also Supplementary Table S4; Supplementary data files S2, S3, S4, S5, S6, S7, S8).
Similar articles
- Structures of the Ets Protein DNA-binding Domains of Transcription Factors Etv1, Etv4, Etv5, and Fev: DETERMINANTS OF DNA BINDING AND REDOX REGULATION BY DISULFIDE BOND FORMATION.
Cooper CD, Newman JA, Aitkenhead H, Allerston CK, Gileadi O. Cooper CD, et al. J Biol Chem. 2015 May 29;290(22):13692-709. doi: 10.1074/jbc.M115.646737. Epub 2015 Apr 12. J Biol Chem. 2015. PMID: 25866208 Free PMC article. - Electrostatic repulsion causes anticooperative DNA binding between tumor suppressor ETS transcription factors and JUN-FOS at composite DNA sites.
Madison BJ, Clark KA, Bhachech N, Hollenhorst PC, Graves BJ, Currie SL. Madison BJ, et al. J Biol Chem. 2018 Nov 30;293(48):18624-18635. doi: 10.1074/jbc.RA118.003352. Epub 2018 Oct 12. J Biol Chem. 2018. PMID: 30315111 Free PMC article. - Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family.
Hollenhorst PC, Shah AA, Hopkins C, Graves BJ. Hollenhorst PC, et al. Genes Dev. 2007 Aug 1;21(15):1882-94. doi: 10.1101/gad.1561707. Epub 2007 Jul 24. Genes Dev. 2007. PMID: 17652178 Free PMC article. - Genomic and biochemical insights into the specificity of ETS transcription factors.
Hollenhorst PC, McIntosh LP, Graves BJ. Hollenhorst PC, et al. Annu Rev Biochem. 2011;80:437-71. doi: 10.1146/annurev.biochem.79.081507.103945. Annu Rev Biochem. 2011. PMID: 21548782 Free PMC article. Review. - ETS transcription factors as emerging drug targets in cancer.
Hsing M, Wang Y, Rennie PS, Cox ME, Cherkasov A. Hsing M, et al. Med Res Rev. 2020 Jan;40(1):413-430. doi: 10.1002/med.21575. Epub 2019 Mar 29. Med Res Rev. 2020. PMID: 30927317 Review.
Cited by
- Heterogeneous dynamics in DNA site discrimination by the structurally homologous DNA-binding domains of ETS-family transcription factors.
He G, Tolic A, Bashkin JK, Poon GM. He G, et al. Nucleic Acids Res. 2015 Apr 30;43(8):4322-31. doi: 10.1093/nar/gkv267. Epub 2015 Mar 30. Nucleic Acids Res. 2015. PMID: 25824951 Free PMC article. - Autoinhibition of ETV6 (TEL) DNA binding: appended helices sterically block the ETS domain.
Coyne HJ 3rd, De S, Okon M, Green SM, Bhachech N, Graves BJ, McIntosh LP. Coyne HJ 3rd, et al. J Mol Biol. 2012 Aug 3;421(1):67-84. doi: 10.1016/j.jmb.2012.05.010. Epub 2012 May 12. J Mol Biol. 2012. PMID: 22584210 Free PMC article. - Mechanisms of in vivo binding site selection of the hematopoietic master transcription factor PU.1.
Pham TH, Minderjahn J, Schmidl C, Hoffmeister H, Schmidhofer S, Chen W, Längst G, Benner C, Rehli M. Pham TH, et al. Nucleic Acids Res. 2013 Jul;41(13):6391-402. doi: 10.1093/nar/gkt355. Epub 2013 May 8. Nucleic Acids Res. 2013. PMID: 23658224 Free PMC article. - Molecular subtyping of primary prostate cancer reveals specific and shared target genes of different ETS rearrangements.
Paulo P, Ribeiro FR, Santos J, Mesquita D, Almeida M, Barros-Silva JD, Itkonen H, Henrique R, Jerónimo C, Sveen A, Mills IG, Skotheim RI, Lothe RA, Teixeira MR. Paulo P, et al. Neoplasia. 2012 Jul;14(7):600-11. doi: 10.1593/neo.12600. Neoplasia. 2012. PMID: 22904677 Free PMC article. - Global analysis of Drosophila Cys₂-His₂ zinc finger proteins reveals a multitude of novel recognition motifs and binding determinants.
Enuameh MS, Asriyan Y, Richards A, Christensen RG, Hall VL, Kazemian M, Zhu C, Pham H, Cheng Q, Blatti C, Brasefield JA, Basciotta MD, Ou J, McNulty JC, Zhu LJ, Celniker SE, Sinha S, Stormo GD, Brodsky MH, Wolfe SA. Enuameh MS, et al. Genome Res. 2013 Jun;23(6):928-40. doi: 10.1101/gr.151472.112. Epub 2013 Mar 7. Genome Res. 2013. PMID: 23471540 Free PMC article.
References
- Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7: 986–995 - PubMed
- Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2: 28–36 - PubMed
- Bartel FO, Higuchi T, Spyropoulos DD (2000) Mouse models in the study of the Ets family of transcription factors. Oncogene 19: 6443–6454 - PubMed
- Batchelor AH, Piper DE, de la Brousse FC, McKnight SL, Wolberger C (1998) The structure of GABPalpha/beta: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science 279: 1037–1041 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous