Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study - PubMed (original) (raw)
Randomized Controlled Trial
. 2010 Oct;105(10):2218-27.
doi: 10.1038/ajg.2010.218. Epub 2010 Jun 1.
Giovanni Brandimarte, Alfredo Papa, Andrea Giglio, Walter Elisei, Gian Marco Giorgetti, Giacomo Forti, Sergio Morini, Cesare Hassan, Maria Antonietta Pistoia, Maria Ester Modeo, Stefano Rodino', Teresa D'Amico, Ladislava Sebkova, Natale Sacca', Emilio Di Giulio, Francesco Luzza, Maria Imeneo, Tiziana Larussa, Salvatore Di Rosa, Vito Annese, Silvio Danese, Antonio Gasbarrini
Affiliations
- PMID: 20517305
- PMCID: PMC3180711
- DOI: 10.1038/ajg.2010.218
Free PMC article
Randomized Controlled Trial
Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study
Antonio Tursi et al. Am J Gastroenterol. 2010 Oct.
Free PMC article
Abstract
Objectives: VSL#3 is a high-potency probiotic mixture that has been used successfully in the treatment of pouchitis. The primary end point of the study was to assess the effects of supplementation with VSL#3 in patients affected by relapsing ulcerative colitis (UC) who are already under treatment with 5-aminosalicylic acid (ASA) and/or immunosuppressants at stable doses.
Methods: A total of 144 consecutive patients were randomly treated for 8 weeks with VSL#3 at a dose of 3,600 billion CFU/day (71 patients) or with placebo (73 patients).
Results: In all, 65 patients in the VSL#3 group and 66 patients in the placebo group completed the study. The decrease in ulcerative colitis disease activity index (UCDAI) scores of 50% or more was higher in the VSL#3 group than in the placebo group (63.1 vs. 40.8; per protocol (PP) P=0.010, confidence interval (CI)₉₅(%) 0.51-0.74; intention to treat (ITT) P=0.031, CI₉₅(%) 0.47-0.69). Significant results with VSL#3 were recorded in an improvement of three points or more in the UCDAI score (60.5% vs. 41.4%; PP P=0.017, CI₉₅(%) 0.51-0.74; ITT P=0.046, CI₉₅(%) 0.47-0.69) and in rectal bleeding (PP P=0.014, CI₉₅(%) 0.46-0.70; ITT P=0.036, CI₉₅(%) 0.41-0.65), whereas stool frequency (PP P=0.202, CI₉₅(%) 0.39-0.63; ITT P=0.229, CI₉₅(%) 0.35-0.57), physician's rate of disease activity (PP P=0.088, CI₉₅(%) 0.34-0.58; ITT P=0.168, CI₉₅(%) 0.31-0.53), and endoscopic scores (PP P=0.086, CI₉₅(%) 0.74-0.92; ITT P=0.366, CI₉₅(%) 0.66-0.86) did not show statistical differences. Remission was higher in the VSL#3 group than in the placebo group (47.7% vs. 32.4%; PP P=0.069, CI₉₅(%) 0.36-0.60; ITT P=0.132, CI₉₅(%) 0.33-0.56). Eight patients on VSL#3 (11.2%) and nine patients on placebo (12.3%) reported mild side effects.
Conclusions: VSL#3 supplementation is safe and able to reduce UCDAI scores in patients affected by relapsing mild-to-moderate UC who are under treatment with 5-ASA and/or immunosuppressants. Moreover, VSL#3 improves rectal bleeding and seems to reinduce remission in relapsing UC patients after 8 weeks of treatment, although these parameters do not reach statistical significance.
Figures
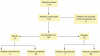
Figure 1
Patient disposition.
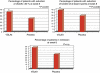
Figure 2
Percentage of patients with reduction of ulcerative colitis disease activity index (UCDAI) > 50% or of at least three points, and patients in remission at week 8 (on intention-to-treat analysis). n.s., not significant.
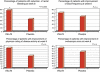
Figure 3
Percentage of patients with improvement in different subgroups of ulcerative colitis disease activity index (UCDAI; rectal bleeding, stool frequency, physician rating of disease activity, and endoscopic score) at week 8 (on intention-to-treat analysis). n.s., not significant.
Comment in
- Treatment with the probiotic VSL#3 as an adjunctive therapy in relapsing mild-to-moderate ulcerative colitis significantly reduces ulcerative colitis disease activity.
Turcotte JF, Huynh HQ. Turcotte JF, et al. Evid Based Med. 2011 Aug;16(4):108-9. doi: 10.1136/ebm1189. Epub 2011 Feb 24. Evid Based Med. 2011. PMID: 21354984 No abstract available. - VSL#3 and remission in active ulcerative colitis: larger studies required.
Jackson EL, Hamlin PJ, Ford AC. Jackson EL, et al. Am J Gastroenterol. 2011 Mar;106(3):547; author reply 547-8. doi: 10.1038/ajg.2010.451. Am J Gastroenterol. 2011. PMID: 21378771 No abstract available. - VSL#3 for ulcerative colitis: growing evidence?
Scott FI, Aberra F. Scott FI, et al. Gastroenterology. 2011 May;140(5):1685-6; discussion 1686-7. doi: 10.1053/j.gastro.2011.03.032. Epub 2011 Mar 25. Gastroenterology. 2011. PMID: 21440588 No abstract available.
Similar articles
- The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis.
Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. Sood A, et al. Clin Gastroenterol Hepatol. 2009 Nov;7(11):1202-9, 1209.e1. doi: 10.1016/j.cgh.2009.07.016. Epub 2009 Jul 22. Clin Gastroenterol Hepatol. 2009. PMID: 19631292 Clinical Trial. - Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis.
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Miele E, et al. Am J Gastroenterol. 2009 Feb;104(2):437-43. doi: 10.1038/ajg.2008.118. Epub 2009 Jan 20. Am J Gastroenterol. 2009. PMID: 19174792 Clinical Trial. - VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases.
Chapman TM, Plosker GL, Figgitt DP. Chapman TM, et al. Drugs. 2006;66(10):1371-87. doi: 10.2165/00003495-200666100-00006. Drugs. 2006. PMID: 16903771 Review. - Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: A systematic review and meta-analysis.
Dang X, Xu M, Liu D, Zhou D, Yang W. Dang X, et al. PLoS One. 2020 Mar 17;15(3):e0228846. doi: 10.1371/journal.pone.0228846. eCollection 2020. PLoS One. 2020. PMID: 32182248 Free PMC article. - Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis.
Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Venturi A, et al. Aliment Pharmacol Ther. 1999 Aug;13(8):1103-8. doi: 10.1046/j.1365-2036.1999.00560.x. Aliment Pharmacol Ther. 1999. PMID: 10468688 Clinical Trial.
Cited by
- Profiling of ABC transporters during active ulcerative colitis and in vitro effect of inflammatory modulators.
Verma N, Ahuja V, Paul J. Verma N, et al. Dig Dis Sci. 2013 Aug;58(8):2282-92. doi: 10.1007/s10620-013-2636-7. Epub 2013 Mar 20. Dig Dis Sci. 2013. PMID: 23512405 - Probiotics in Inflammatory Bowel Disease: Are We Back to Square One?
Puvvada SR, Luvsannyam E, Patel D, Hassan Z, Hamid P. Puvvada SR, et al. Cureus. 2020 Sep 4;12(9):e10247. doi: 10.7759/cureus.10247. Cureus. 2020. PMID: 33042686 Free PMC article. Review. - Enterococcus durans TN-3 Induces Regulatory T Cells and Suppresses the Development of Dextran Sulfate Sodium (DSS)-Induced Experimental Colitis.
Kanda T, Nishida A, Ohno M, Imaeda H, Shimada T, Inatomi O, Bamba S, Sugimoto M, Andoh A. Kanda T, et al. PLoS One. 2016 Jul 20;11(7):e0159705. doi: 10.1371/journal.pone.0159705. eCollection 2016. PLoS One. 2016. PMID: 27438072 Free PMC article. - Effects of Probiotic Supplementation on Short Chain Fatty Acids in the AppNL-G-F Mouse Model of Alzheimer's Disease.
Kaur H, Golovko S, Golovko MY, Singh S, Darland DC, Combs CK. Kaur H, et al. J Alzheimers Dis. 2020;76(3):1083-1102. doi: 10.3233/JAD-200436. J Alzheimers Dis. 2020. PMID: 32623399 Free PMC article. - Therapeutic Effects of Zymomonas mobilis on Experimental DSS-Induced Colitis Mouse Model.
Almo MMD, Sousa IG, Olinto VG, Pinhate SB, Jivago JLPR, de Sousa DER, Castro MB, Rubini MR, Maranhão AQ, Brigido MM. Almo MMD, et al. Microorganisms. 2023 Nov 17;11(11):2793. doi: 10.3390/microorganisms11112793. Microorganisms. 2023. PMID: 38004805 Free PMC article.
References
- Schwartz M, Cohen R. Optimizing conventional therapy for inflammatory bowel disease. Curr Gastroenterol Rep. 2008;10:585–590. - PubMed
- Travis S, Stange EF, Lémann M, et al. For the European Crohn's and Colitis Organisation (ECCO). European evidence-based consensus of the management of ulcerative colitis: current management. J Crohn Colitis. 2008;2:24–62. - PubMed
- Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. - PubMed
- Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. - PubMed
- Tursi A, Brandimarte G, Giorgetti GM, et al. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10:PI126–PI131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous