Mitochondrial dynamics in diabetes - PubMed (original) (raw)
Review
Mitochondrial dynamics in diabetes
Yisang Yoon et al. Antioxid Redox Signal. 2011.
Abstract
Mitochondria are at the center of cellular energy metabolism and regulate cell life and death. The cell biological aspect of mitochondria, especially mitochondrial dynamics, has drawn much attention through implications in human pathology, including neurological disorders and metabolic diseases. Mitochondrial fission and fusion are the main processes governing the morphological plasticity and are controlled by multiple factors, including mechanochemical enzymes and accessory proteins. Emerging evidence suggests that mitochondrial dynamics plays an important role in metabolism-secretion coupling in pancreatic β-cells as well as complications of diabetes. This review describes an overview of mechanistic and functional aspects of mitochondrial fission and fusion, and comments on the recent advances connecting mitochondrial dynamics with diabetes and diabetic complications.
Figures
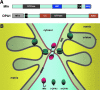
FIG. 1.
Dynamic mitochondria. (A) Network organization of mitochondria. Mitochondria are labeled with the matrix-targeted green fluorescent protein. (B, C) Individual images from time-lapse imaging of mitochondria showing fission and fusion. Images are inverted for better observation of fission and fusion. Images in (B) show two successive fission events in one mitochondrial tubule (arrows). Arrowheads in (C) denote two fusion events. N, nucleus.
FIG. 2.
Control of fission/fusion balance. (A) Quantification of fission and fusion frequencies from time-lapse sequences acquired in every 10 s for 100 frames. Time-lapse images from normal, nocodazole-treated, and dynamin-like protein 1 (DLP1)-K38A mutant cells were subjected to the quantification. Fission events were counted when a single mitochondrion became two by a spatial separation that sustained at least two consecutive frames. Similarly, fusion was counted when separate mitochondria became converged and the connection sustained at least for two consecutive frames. Total number of fission and fusion were divided by the number of mitochondria that were present at the initial frame of the time-lapse sequence, and the frequency of fission and fusion was expressed as the events/hour/mitochondrion. Three regions were selected within a cell and the average fission and fusion were calculated. The quantification was repeated in a total of three cells for each treatment. The frequency of fission is similar to that of fusion within each region (∼5–6 events/hour/mito), demonstrating a balance between fission and fusion. Nocodazole treatment did not change the fission and fusion frequency, indicating that microtubules are not essential for these processes. Expression of DLP1-K38A resulted in a twofold decrease in both fission and fusion. (B) A proposed feedback mechanism to maintain a balance between mitochondrial fission and fusion. Frequency of mitochondrial fusion is predicted to be proportional to the number of mitochondrial tips. Likewise, the number of fission events is likely to increase as tubule length increases. In cells with reduced fission (e.g., DLP1-K38A expressing cells), fusion frequency also decreased due to the decrease of the number of mitochondrial tips available for fusion (A). In healthy cells with properly working fission and fusion machineries, the elongation of mitochondrial tubules due to increased fusion would increase fission frequency, which rapidly restores normal mitochondrial shape.
FIG. 3.
Mitochondrial fission. (A) Mitochondrial fission proteins DLP1 and fission protein 1 (Fis1). The guanosine triphosphate (GTP)ase domain of DLP1 is highly conserved to that of conventional dynamin, while the middle and coiled-coil (CC) domain are moderately conserved. The CC domain is also known as GTPase effector domain (GED). Unconserved (UC) domain shows no homology to other dynamin proteins. Two red-colored boxes in N- and C-termini denote alternative splicing regions. Fis1 is a small protein containing a single transmembrane (TM) domain. The middle four helices form tetratricopeptide repeat (TPR) motifs. (B) Liposome tubulation by DLP1. Incubation of spherical phosphatidylserine liposomes (left) with purified recombinant DLP1 in the presence of GTPγS induces the tubule formation (right). (C) Mitochondrial fission: DLP1 forms a ring-like structure around the mitochondrial tubule with or without the aid of Fis1. DLP1 constricts and divides the mitochondrial tubule through GTP hydrolysis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
FIG. 4.
Mitochondrial fusion. (A) Mitochondrial fusion proteins mitofusin (Mfn) and optic atrophy 1 (OPA1). See text for the structural description. MTS, mitochondrial targeting sequence. (B) Mitochondrial tethering and fusion. The interaction between C-terminal heptad repeat 2 (HR2) domains of Mfn in adjacent mitochondria tethers mitochondria, and membrane fusion ensues. Inner membrane fusion requires both long and short forms of OPA1 (l- and s-OPA1). OPA1 also regulates the size of the cristae junction. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
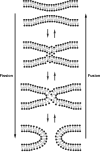
FIG. 5.
Fission and fusion of membrane bilayers. Mixing of lipid molecules in the two contacting bilayers form a hemifusion state before complete reorganization into new bilayers.
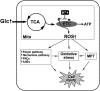
FIG. 6.
Mitochondrial reactive oxygen species (ROS) generation in hyperglycemia. High glucose concentrations increase metabolic input into mitochondria. Hyperpolarized mitochondria slow down electron flow through the electron transport chain (ETC) and increase superoxide (ROS) generation. High ROS levels cause the mitochondrial permeability transition (MPT), oxidative stresses, and the increase of other harmful metabolic/signaling pathways, leading to cell injury.
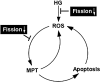
FIG. 7.
A vicious cycle of ROS and apoptosis during hyperglycemic insult. Hyperglycemia (HG) increases ROS through mitochondrial fragmentation. Increased ROS induces MPT, initiating apoptotic cascade. Cytochrome c dislocation and caspase activation further enhance ROS production through ETC dysfunction. We postulate that mitochondrial fission participates in both HG-induced mitochondrial fragmentation and ROS-induced MPT/apoptosis. Therefore, inhibiting mitochondrial fission would diminish ROS toxicity by acting at initial ROS generation and/or ROS amplification in HG.
FIG. 8.
Life cycle of mitochondria. Mitochondrial biogenesis requires new syntheses and transport of proteins and lipids. Old and dysfunctional mitochondria are eliminated by the autophagic process. Between their birth and death, mitochondria carry out essential cellular processes, which requires proper maintenance of mitochondrial activity. Through a spatio-temporal regulation of shape and location (mitochondrial dynamics), mitochondria maximize their functional efficiency and life span. Therefore, mitochondrial homeostasis, including biogenesis, dynamics, and autophagic removal, needs to be regulated in a coordinated manner, and dysregulation of any of these processes leads to mitochondrial dysfunction and disease conditions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
Similar articles
- Mitochondrial morphology in metabolic diseases.
Galloway CA, Yoon Y. Galloway CA, et al. Antioxid Redox Signal. 2013 Aug 1;19(4):415-30. doi: 10.1089/ars.2012.4779. Epub 2012 Aug 27. Antioxid Redox Signal. 2013. PMID: 22793999 Free PMC article. Review. - Role of mitofusin 2 in cardiovascular oxidative injury.
Zheng M, Xiao RP. Zheng M, et al. J Mol Med (Berl). 2010 Oct;88(10):987-91. doi: 10.1007/s00109-010-0675-5. Epub 2010 Sep 8. J Mol Med (Berl). 2010. PMID: 20824264 Review. - Mitochondrial morphology transitions and functions: implications for retrograde signaling?
Picard M, Shirihai OS, Gentil BJ, Burelle Y. Picard M, et al. Am J Physiol Regul Integr Comp Physiol. 2013 Mar 15;304(6):R393-406. doi: 10.1152/ajpregu.00584.2012. Epub 2013 Jan 30. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23364527 Free PMC article. Review. - Mitochondria and diabetes. An intriguing pathogenetic role.
Newsholme P, Gaudel C, Krause M. Newsholme P, et al. Adv Exp Med Biol. 2012;942:235-47. doi: 10.1007/978-94-007-2869-1_10. Adv Exp Med Biol. 2012. PMID: 22399425 Review. - Mitochondrial fusion proteins: dual regulators of morphology and metabolism.
Zorzano A, Liesa M, Sebastián D, Segalés J, Palacín M. Zorzano A, et al. Semin Cell Dev Biol. 2010 Aug;21(6):566-74. doi: 10.1016/j.semcdb.2010.01.002. Epub 2010 Jan 15. Semin Cell Dev Biol. 2010. PMID: 20079867 Review.
Cited by
- Mechanisms for countering oxidative stress and damage in retinal pigment epithelium.
Plafker SM, O'Mealey GB, Szweda LI. Plafker SM, et al. Int Rev Cell Mol Biol. 2012;298:135-77. doi: 10.1016/B978-0-12-394309-5.00004-3. Int Rev Cell Mol Biol. 2012. PMID: 22878106 Free PMC article. Review. - A mitochondria-targeted mass spectrometry probe to detect glyoxals: implications for diabetes.
Pun PB, Logan A, Darley-Usmar V, Chacko B, Johnson MS, Huang GW, Rogatti S, Prime TA, Methner C, Krieg T, Fearnley IM, Larsen L, Larsen DS, Menger KE, Collins Y, James AM, Kumar GD, Hartley RC, Smith RA, Murphy MP. Pun PB, et al. Free Radic Biol Med. 2014 Feb;67(100):437-50. doi: 10.1016/j.freeradbiomed.2013.11.025. Epub 2013 Dec 4. Free Radic Biol Med. 2014. PMID: 24316194 Free PMC article. - The dynamin-related protein DRP-1 and the insulin signaling pathway cooperate to modulate Caenorhabditis elegans longevity.
Yang CC, Chen D, Lee SS, Walter L. Yang CC, et al. Aging Cell. 2011 Aug;10(4):724-8. doi: 10.1111/j.1474-9726.2011.00711.x. Epub 2011 Apr 25. Aging Cell. 2011. PMID: 21463460 Free PMC article. - Zinc cytotoxicity induces mitochondrial morphology changes in hela cell line.
Knies KA, Li YV. Knies KA, et al. Int J Physiol Pathophysiol Pharmacol. 2021 Apr 15;13(2):43-51. eCollection 2021. Int J Physiol Pathophysiol Pharmacol. 2021. PMID: 34093965 Free PMC article. - Mitochondrial fragmentation and network architecture in degenerative diseases.
Shah SI, Paine JG, Perez C, Ullah G. Shah SI, et al. PLoS One. 2019 Sep 26;14(9):e0223014. doi: 10.1371/journal.pone.0223014. eCollection 2019. PLoS One. 2019. PMID: 31557225 Free PMC article.
References
- Alexander C. Votruba M. Pesch UE. Thiselton DL. Mayer S. Moore A. Rodriguez M. Kellner U. Leo-Kottler B. Auburger G, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. - PubMed
- Anello M. Lupi R. Spampinato D. Piro S. Masini M. Boggi U. Del Prato S. Rabuazzo AM. Purrello F. Marchetti P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–289. - PubMed
- Arnoult D. Rismanchi N. Grodet A. Roberts RG. Seeburg DP. Estaquier J. Sheng M. Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr Biol. 2005;15:2112–2118. - PubMed
- Autret A. Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 2009;36:355–363. - PubMed
- Bach D. Naon D. Pich S. Soriano FX. Vega N. Rieusset J. Laville M. Guillet C. Boirie Y. Wallberg-Henriksson H, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical