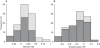Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission - PubMed (original) (raw)
Review
Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission
Louis Lambrechts et al. PLoS Negl Trop Dis. 2010.
Abstract
The dramatic global expansion of Aedes albopictus in the last three decades has increased public health concern because it is a potential vector of numerous arthropod-borne viruses (arboviruses), including the most prevalent arboviral pathogen of humans, dengue virus (DENV). Ae. aegypti is considered the primary DENV vector and has repeatedly been incriminated as a driving force in dengue's worldwide emergence. What remains unresolved is the extent to which Ae. albopictus contributes to DENV transmission and whether an improved understanding of its vector status would enhance dengue surveillance and prevention. To assess the relative public health importance of Ae. albopictus for dengue, we carried out two complementary analyses. We reviewed its role in past dengue epidemics and compared its DENV vector competence with that of Ae. aegypti. Observations from "natural experiments" indicate that, despite seemingly favorable conditions, places where Ae. albopictus predominates over Ae. aegypti have never experienced a typical explosive dengue epidemic with severe cases of the disease. Results from a meta-analysis of experimental laboratory studies reveal that although Ae. albopictus is overall more susceptible to DENV midgut infection, rates of virus dissemination from the midgut to other tissues are significantly lower in Ae. albopictus than in Ae. aegypti. For both indices of vector competence, a few generations of mosquito colonization appear to result in a relative increase of Ae. albopictus susceptibility, which may have been a confounding factor in the literature. Our results lead to the conclusion that Ae. albopictus plays a relatively minor role compared to Ae. aegypti in DENV transmission, at least in part due to differences in host preferences and reduced vector competence. Recent examples of rapid arboviral adaptation to alternative mosquito vectors, however, call for cautious extrapolation of our conclusion. Vector status is a dynamic process that in the future could change in epidemiologically important ways.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Distribution of RD among published experiments comparing the vector competence of Ae. albopictus and Ae. aegypti for horizontal transmission of DENV.
Graphs show the overall frequency of differences in (A) the proportion of infected mosquitoes and (B) the proportion of mosquitoes with an infection disseminated from the midgut, as a function of the mosquito colonization history (i.e., number of generations spent in the laboratory before vector competence was assessed). Filled bars represent mosquitoes held ≤5 generations in the laboratory; shaded bars correspond to mosquitoes colonized for >5 generations. Negative RD values represent a reduced rate whereas positive values represent a greater rate for Ae. albopictus compared to Ae. aegypti.
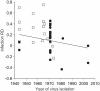
Figure 2. Relationship between RD and virus isolation year among published experiments comparing DENV midgut infection in Ae. albopictus and Ae. aegypti.
Each point represents a single experiment. Different symbols indicate a different mosquito colonization history (i.e., number of generations spent in the laboratory before vector competence was assessed). Filled circles represent mosquitoes held ≤5 generations in the laboratory; open squares correspond to mosquitoes colonized for >5 generations. The solid line shows the linear regression (R2 = 0.162, P = 0.007). Negative RD values represent a reduced rate whereas positive values represent a greater rate for Ae. albopictus compared to Ae. aegypti.
Similar articles
- Risk of dengue in Central Africa: Vector competence studies with Aedes aegypti and Aedes albopictus (Diptera: Culicidae) populations and dengue 2 virus.
Kamgang B, Vazeille M, Tedjou AN, Wilson-Bahun TA, Yougang AP, Mousson L, Wondji CS, Failloux AB. Kamgang B, et al. PLoS Negl Trop Dis. 2019 Dec 30;13(12):e0007985. doi: 10.1371/journal.pntd.0007985. eCollection 2019 Dec. PLoS Negl Trop Dis. 2019. PMID: 31887138 Free PMC article. - Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission.
Brady OJ, Golding N, Pigott DM, Kraemer MU, Messina JP, Reiner RC Jr, Scott TW, Smith DL, Gething PW, Hay SI. Brady OJ, et al. Parasit Vectors. 2014 Jul 22;7:338. doi: 10.1186/1756-3305-7-338. Parasit Vectors. 2014. PMID: 25052008 Free PMC article. - Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in the Florida Keys.
Richards SL, Anderson SL, Alto BW. Richards SL, et al. J Med Entomol. 2012 Jul;49(4):942-6. doi: 10.1603/me11293. J Med Entomol. 2012. PMID: 22897056 - [Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control].
Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. Delatte H, et al. Parasite. 2008 Mar;15(1):3-13. doi: 10.1051/parasite/2008151003. Parasite. 2008. PMID: 18416242 Review. French. - Critical review of the vector status of Aedes albopictus.
Gratz NG. Gratz NG. Med Vet Entomol. 2004 Sep;18(3):215-27. doi: 10.1111/j.0269-283X.2004.00513.x. Med Vet Entomol. 2004. PMID: 15347388 Review.
Cited by
- Neurological sequelae induced by alphavirus infection of the CNS are attenuated by treatment with the glutamine antagonist 6-diazo-5-oxo-l-norleucine.
Potter MC, Baxter VK, Mathey RW, Alt J, Rojas C, Griffin DE, Slusher BS. Potter MC, et al. J Neurovirol. 2015 Apr;21(2):159-73. doi: 10.1007/s13365-015-0314-6. Epub 2015 Feb 3. J Neurovirol. 2015. PMID: 25645378 Free PMC article. - Host-feeding pattern of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India.
Sivan A, Shriram AN, Sunish IP, Vidhya PT. Sivan A, et al. Parasitol Res. 2015 Sep;114(9):3539-46. doi: 10.1007/s00436-015-4634-5. Epub 2015 Jul 30. Parasitol Res. 2015. PMID: 26220560 - Aedes (Stegomyia) albopictus' dynamics influenced by spatiotemporal characteristics in a Brazilian dengue-endemic risk city.
Bezerra JMT, Araújo RGP, Melo FF, Gonçalves CM, Chaves BA, Silva BM, Silva LD, Brandão ST, Secundino NFC, Norris DE, Pimenta PFP. Bezerra JMT, et al. Acta Trop. 2016 Dec;164:431-437. doi: 10.1016/j.actatropica.2016.10.010. Epub 2016 Oct 19. Acta Trop. 2016. PMID: 27771419 Free PMC article. - Dengue on islands: a Bayesian approach to understanding the global ecology of dengue viruses.
Feldstein LR, Brownstein JS, Brady OJ, Hay SI, Johansson MA. Feldstein LR, et al. Trans R Soc Trop Med Hyg. 2015 May;109(5):303-12. doi: 10.1093/trstmh/trv012. Epub 2015 Mar 13. Trans R Soc Trop Med Hyg. 2015. PMID: 25771261 Free PMC article. Clinical Trial. - Modeling dengue vector dynamics under imperfect detection: three years of site-occupancy by Aedes aegypti and Aedes albopictus in urban Amazonia.
Padilla-Torres SD, Ferraz G, Luz SL, Zamora-Perea E, Abad-Franch F. Padilla-Torres SD, et al. PLoS One. 2013;8(3):e58420. doi: 10.1371/journal.pone.0058420. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472194 Free PMC article.
References
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. - PubMed
- Mitchell CJ. The role of Aedes albopictus as an arbovirus vector. Parassitologia. 1995;37:109–113. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous