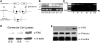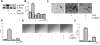Inhibition of podocyte FAK protects against proteinuria and foot process effacement - PubMed (original) (raw)
. 2010 Jul;21(7):1145-56.
doi: 10.1681/ASN.2009090991. Epub 2010 Jun 3.
Akashi Togawa, Keita Soda, Junhui Zhang, Sik Lee, Ming Ma, Zhiheng Yu, Thomas Ardito, Jan Czyzyk, Lonnette Diggs, Dominique Joly, Shinji Hatakeyama, Eiji Kawahara, Lawrence Holzman, Jun Lin Guan, Shuta Ishibe
Affiliations
- PMID: 20522532
- PMCID: PMC3152231
- DOI: 10.1681/ASN.2009090991
Inhibition of podocyte FAK protects against proteinuria and foot process effacement
Hong Ma et al. J Am Soc Nephrol. 2010 Jul.
Abstract
Focal adhesion kinase (FAK) is a nonreceptor tyrosine kinase that plays a critical role in cell motility. Movement and retraction of podocyte foot processes, which accompany podocyte injury, suggest focal adhesion disassembly. To understand better the mechanisms by which podocyte foot process effacement leads to proteinuria and kidney failure, we studied the function of FAK in podocytes. In murine models, glomerular injury led to activation of podocyte FAK, followed by proteinuria and foot process effacement. Both podocyte-specific deletion of FAK and pharmacologic inactivation of FAK abrogated the proteinuria and foot process effacement induced by glomerular injury. In vitro, podocytes isolated from conditional FAK knockout mice demonstrated reduced spreading and migration; pharmacologic inactivation of FAK had similar effects on wild-type podocytes. In conclusion, FAK activation regulates podocyte foot process effacement, suggesting that pharmacologic inhibition of this signaling cascade may have therapeutic potential in the setting of glomerular injury.
Figures
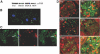
Figure 1.
Podocyte injury activates FAK. (A) Whole-cell lysates from podocytes and IMCD, MCDK, and MPT cells immunoblotted with α-FAK. (B) Stable differentiated podocytes stained with α-FAK (green) to assess for focal adhesions. Arrow indicates focal adhesion complex. (C) Kidney cryosections stained with α-FAK (arrow, green), α-WT-1 (red), and merge. (D) Representative glomeruli from LPS-treated FAK+/+;Pod-Cre mice at the indicated time points stained with α-pFAK Y397 (green; arrowhead at 6 hours) and with α-WT-1 (red; arrow at 6 hours). The bottom two panels represent representative glomeruli from FAK+/+;Pod-Cre mice treated with rabbit anti-mouse GBM at day 6 stained with antibodies as above (arrows indicating pFAKY397).
Figure 2.
FAK is specifically deleted in podocytes. (A) Algorithm used to generate podocyte-specific FAK knockout mice (primer designated A and B). (B) Representative PCR analysis of extracted genomic DNA from mouse-tail clippings from _FAK_fl/fl;_Pod_-Cre (1600 bp), FAK+/+;_Pod_-Cre (1500 bp), and FAK fl/+;
_Pod_-Cre. (C) Representative PCR analysis of extracted genomic DNA assessing tissue specific excision of FAK. Controls from tail (tl), heart (ht), liver, spleen (spl), and lung (lg) reveal an approximately 1.6-kb band that represents the nonexcised FAK gene product. The tissue-specific deletion of the loxP-flanked sequence of the podocytes of the renal cortex results in the expected 600-bp fragment (arrow) as well as a 1.6-kb product representing nonexcised genomic DNA, presumed mainly from the proximal tubule cells in this region (cortex) of the kidney. (D) Western blot analysis of glomerular cell lysates obtained from two FAK fl /fl;Pod -Cre mice and one FAK+/+;Pod-Cre mouse immunoblotted with α-FAK and α-β-actin. (E) Representative Western blot from two FAK fl/fl;Pod-Cre mouse primary podocytes (left two lanes) and two FAK+/+;Pod-Cre mice (two right lanes) immunoblotted with α-FAK, α-podocin, and α-paxillin.
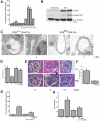
Figure 3.
Deletion of FAK in podocytes diminishes podocyte injury and proteinuria. (A) ACR from FAK fl/fl;Pod-Cre and FAK+/+;Pod-Cre mice after LPS at indicated time points. ACR measured from samples at 0, 12, 24, 36, and 72 hours (n = 8). *P < 0.001 at 12 hours; **P < 0.001 at 24 hours; ***P < 0.001 at 36 hours. (B) Representative Western blot from isolated primary podocyte cell lysates from FAK fl/fl;Pod-Cre and FAK+/+;
Pod-Cre mice stimulated with or without LPS and immunoblotted with α-FAK, α-pFAK 397, and α-α-tubulin. (C) Representative electron microscopy image of FAK fl/fl; Pod-Cre and FAK+/+;P od-Cre mouse podocytes stimulated with or without LPS. (D) Quantification of C (n = 3). ∧P < 0.01. (E) Representative hematoxylin and eosin staining of glomeruli from FAK+/+;Pod-Cre and FAK fl/fl;Pod-Cre mouse after rabbit anti-mouse GBM treatment at the indicated time points. (F) Quantification of glomerular crescent formation (expressed as percentage of 25 glomeruli examined per mouse) (n = 5, #P < 0.005). (G) ACR at day 6 after injection of α-rabbit-anti-mouse GBM in FAK fl/fl;Pod-Cre and FAK+/+;Pod-Cre mice (n = 7). ∧P < 0.01. (H) Serum creatinine at day 6 after injection of α-rabbit-anti-mouse GBM in FAK fl/fl;Pod-Cre and FAK+/+;Pod-Cre mice (n = 7). &P < 0.05.
Figure 4.
FAK fl/fl;Pod-Cre mouse primary podocytes are resistant to LPS stimulated migration. (A) Representative Western blot from FAK+/+;Pod-Cre primary podocytes with or without LPS immunoblotted with α-pFAK Y397 and α-FAK at the indicated time points. (B) Quantification of fold increase by densitometry (n = 3). *P < 0.001; ∧P < 0.01. (C) Quantification of adhesion assay (n = 4). (D) Representative image of cell spreading of primary FAK+/+;Pod-Cre and FAK fl/fl;Pod-Cre podocytes stimulated with or without LPS. (E) Podocytes were pretreated with or without LPS for 6 hours and plated on Transwell filters and allowed to attach for 6 hours, and cells that had migrated to the bottom of the Transwell in 12 hours were photographed. Cell migration was scored positive when the nuclei were visible. (F) Quantification of E (n = 3). *P < 0.001 FAK+/+;
Pod-Cre (WT) versus FAK fl/fl;Pod-Cre (FAK null) + LPS. (G) Wound-healing assay from podocytes isolated in FAK fl/fl;Pod-Cre and FAK+/+;Pod-Cre mice with or without LPS. (H) Quantification of G (n = 4). *P < 0.001 FAK+/+;Pod-Cre (WT) versus FAK fl/fl;Pod-Cre (FAK null) + LPS. Magnifications: ×200 in D; ×400 in G.
Figure 5.
FAK inhibitor TAE-226 inhibits podocyte FAK activation and migration. (A) Primary podocytes treated with or without TAE-226 and with or without LPS immunoblotted with α-FAK and α-pFAK Y397. (B) Quantification of cell-spreading assay in podocytes isolated from FAK fl/fl;
Pod-Cre and FAK+/+;Pod-Cre mice treated with or without LPS and with or without TAE-226 (n = 3). *P < 0.001; lane 2 versus lane 4 or lane 6. (C) Podocytes treated with or without TAE-226 were seeded on Transwell filters for 6 hours and treated with or without LPS, and cells that migrated to the bottom were photographed. (D) Quantification of C (n = 4). ∧P < 0.01. (E) Wound-healing assay from WT primary podocytes treated with or without LPS and with or without TAE-226. (F) Quantification of E (n = 4). #P < 0.005. Magnification, ×400.
Figure 6.
Loss of FAK results in increased stress fiber formation and activation of Rho. (A) Podocytes harvested from isolated glomeruli from FAK+/+;
Pod-Cre and FAK fl/fl;Pod-Cre mice (designated as WT, FAK KO, and TAE-226) treated with or without LPS were immunostained with α-phalloidin and TOPRO (nuclear marker). (B) FAK KD and WT immortalized podocytes treated with or without LPS for 6 hours and immunoblotted with α-paxillin and α-p-paxillin (pS83). (C) FAK KD and WT podocytes treated with or without LPS and incubated with agarose-PAK and immunoblotted with α-Rac. (D) FAK KD and WT podocytes treated with or without LPS and incubated with agarose-Rhotekin RBD and immunoblotted with α-Rho. (E) WT, KO, FAK RC, and KO podocytes pretreated with Rho associated kinase inhibitor Y27632 (1 μm) stimulated with LPS were immunostained with α-phalloidin and TOPRO (nuclear marker). (F) Quantification of percentage of stress fiber formation from E after LPS stimulation (n = 4). &P < 0.05, lane 2 versus lane 4 or 5.
Figure 7.
TAE-226 treatment reduces proteinuria and podocyte injury. (A) ACR from mice pretreated with or without TAE-226 and with or without LPS at the indicated time points (n = 9). #P < 0.005; ∧P < 0.01. (B) Representative electron microscopy image of WT mice treated with or without TAE-226 and with or without LPS; arrow indicates podocyte effacement (left). (C) Quantification of C (n = 3). P < 0.001. (D) ACR in mice treated at time of injury with or without TAE 226 and with or without rabbit anti-mouse antibody (n = 6). *P < 0.001. (E) Serum creatinine obtained from mice pretreated with or without TAE-226, before injection of rabbit anti-mouse GBM antibody (first four bars). Serum creatinine from mice that were treated with or without TAE-226 at the time of injection of rabbit anti-mouse GBM antibody (last two bars; n = 6). #P < 0.005; ∧P < 0.01. (F) Serum creatinine from mice treated with or without TAE 226 after injury with rabbit anti-mouse GBM antibody (n = 4).
Similar articles
- Protein tyrosine phosphatase 1B inhibition protects against podocyte injury and proteinuria.
Kumagai T, Baldwin C, Aoudjit L, Nezvitsky L, Robins R, Jiang R, Takano T. Kumagai T, et al. Am J Pathol. 2014 Aug;184(8):2211-24. doi: 10.1016/j.ajpath.2014.05.005. Epub 2014 Jun 18. Am J Pathol. 2014. PMID: 24951831 - FHL2 mediates podocyte Rac1 activation and foot process effacement in hypertensive nephropathy.
Li SY, Chu PH, Huang PH, Hsieh TH, Susztak K, Tarng DC. Li SY, et al. Sci Rep. 2019 Apr 30;9(1):6693. doi: 10.1038/s41598-019-42328-1. Sci Rep. 2019. PMID: 31040292 Free PMC article. - IL-1 receptor signaling in podocytes limits susceptibility to glomerular damage.
Ren J, Lu X, Hall G, Privratsky JR, Robson MJ, Blakely RD, Crowley SD. Ren J, et al. Am J Physiol Renal Physiol. 2022 Feb 1;322(2):F164-F174. doi: 10.1152/ajprenal.00353.2021. Epub 2021 Dec 13. Am J Physiol Renal Physiol. 2022. PMID: 34894725 Free PMC article. - Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases.
Sever S, Schiffer M. Sever S, et al. Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review. - A Review of Podocyte Biology.
Garg P. Garg P. Am J Nephrol. 2018;47 Suppl 1:3-13. doi: 10.1159/000481633. Epub 2018 May 31. Am J Nephrol. 2018. PMID: 29852492 Review.
Cited by
- DOT1L protects against podocyte injury in diabetic kidney disease through phospholipase C-like 1.
Hu Y, Ye S, Kong J, Zhou Q, Wang Z, Zhang Y, Yan H, Wang Y, Li T, Xie Y, Chen B, Zhao Y, Zhang T, Zheng X, Niu J, Hu B, Wang S, Chen Z, Zheng C. Hu Y, et al. Cell Commun Signal. 2024 Oct 25;22(1):519. doi: 10.1186/s12964-024-01895-1. Cell Commun Signal. 2024. PMID: 39456056 Free PMC article. - IGFBP2 induces podocyte apoptosis promoted by mitochondrial damage via integrin α5/FAK in diabetic kidney disease.
Wang X, Zhang Y, Chi K, Ji Y, Zhang K, Li P, Fu Z, Wang X, Cui S, Shen W, Cai G, Chen X, Zhu H, Hong Q. Wang X, et al. Apoptosis. 2024 Aug;29(7-8):1109-1125. doi: 10.1007/s10495-024-01974-1. Epub 2024 May 25. Apoptosis. 2024. PMID: 38796567 - Pharmacological Blockade of the Adenosine A2B Receptor Is Protective of Proteinuria in Diabetic Rats, through Affecting Focal Adhesion Kinase Activation and the Adhesion Dynamics of Podocytes.
Mendoza-Soto P, Jara C, Torres-Arévalo Á, Oyarzún C, Mardones GA, Quezada-Monrás C, San Martín R. Mendoza-Soto P, et al. Cells. 2024 May 16;13(10):846. doi: 10.3390/cells13100846. Cells. 2024. PMID: 38786068 Free PMC article. - Roles of myosin 1e and the actin cytoskeleton in kidney functions and familial kidney disease.
Liu PJ, Sayeeda K, Zhuang C, Krendel M. Liu PJ, et al. Cytoskeleton (Hoboken). 2024 May 6:10.1002/cm.21861. doi: 10.1002/cm.21861. Online ahead of print. Cytoskeleton (Hoboken). 2024. PMID: 38708443 Review. - The spatially resolved transcriptome signatures of glomeruli in chronic kidney disease.
Clair G, Soloyan H, Cravedi P, Angeletti A, Salem F, Al-Rabadi L, De Filippo RE, Da Sacco S, Lemley KV, Sedrakyan S, Perin L. Clair G, et al. JCI Insight. 2024 Mar 22;9(6):e165515. doi: 10.1172/jci.insight.165515. JCI Insight. 2024. PMID: 38516889 Free PMC article.
References
- Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 - PubMed
- Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 - PubMed
- Patrakka J, Kestila M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Mannikko M, Visapaa I, Holmberg C, Rapola J, Tryggvason K, Jalanko H: Congenital nephrotic syndrome (NPHS1): Features resulting from different mutations in Finnish patients. Kidney Int 58: 972–980, 2000 - PubMed
- Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous