Targeting Hedgehog--a cancer stem cell pathway - PubMed (original) (raw)
Review
Targeting Hedgehog--a cancer stem cell pathway
Akil A Merchant et al. Clin Cancer Res. 2010.
Abstract
The Hedgehog (Hh) pathway has been implicated in a wide variety of human tumors, and early clinical trials with pathway antagonists have validated Hh signaling as a bona fide anticancer target. Despite these encouraging results, several issues surrounding the basic biology of the Hh pathway in human cancers remain unclear. These include the influence of specific oncogenic events on Hh signal transduction, the precise mode of Hh signaling (i.e., autocrine or paracrine) that occurs within human tumors, and the best means to inhibit aberrant pathway activity in the clinical setting. The cancer stem cell (CSC) hypothesis may explain a number of clinical phenomena, such as unchecked self-renewal and the development of metastatic disease, and to some extent, the Hh signaling pathway has been implicated in all of these processes. Therefore, Hh pathway inhibitors may also represent some of the first agents to formally examine the CSC hypothesis in the clinical setting. The diverse nature of Hh signaling in human cancers suggests that disease-specific factors must be carefully considered to identify the optimal use of novel pathway inhibitors.
(c) 2010 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
W. Matsui, commercial research grant, Merck; patent interest, Infinity Pharmaceuticals; consultant, Bristol-Myers Squibb, Pfizer.
Figures
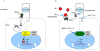
Figure 1. Hedgehog signaling
A schematic of Hh pathway signal transduction derived from developmental and cancer models. (A) In the absence of Hh ligand, Ptch is located in the cilium and blocks Smo entry. Gli transcription factors exist in repressor forms that prevent transcription of target genes. (B) Three mammalian homologues of Hh (SHh, IHh, DHh) bind Ptch at the cell surface and allow it to move out of the primary cilium. Smo is derepressed and moves into the primary cilium where it can activate Gli transcription factors. During this process, the Gli transcription factors are processed to activator forms and translocated to the nucleus to induce the transcription of Hh target genes. Antibodies against the Hh ligands (5E1) and robotnikinin block pathway activation by preventing the interaction of Hh ligand with Ptch. Cyclopamine and novel antagonists of Smo directly bind and inhibit its function. Compounds such as HPI 2,3,4 block the transport of components in the signaling cascade. Direct Gli antagonists such as GANT block binding of Gli transcription factors to DNA.
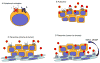
Figure 2. Models of Hh signaling in cancer
(A) Non-ligand mediated signaling (mutational activation). Mutations in PTCH1, SMO, SUFU or amplification of GLI1 have all been reported in human cacners. i. Loss of PTCH1 activity may increase Hh pathway activity. ii. Overexpression or activating mutations in SMO are also depicted. (B) Ligand mediated autocrine signaling. Tumor cells produce Hh ligand that stimulates Hh pathway activity in tumor cells. (C) Ligand mediated paracrine signaling. Non-malignant stromal cells produces Hh ligand required by tumor cells for growth and survival. (D) Ligand mediated paracrine signaling. Tumor cells produce Hh ligand that activates Hh signaling in non-malignant stromal and endothelial cells. This results in the production of unknown factors within the microenvironment that ultimately supports tumor cell growth and survival as well as angiogenesis.
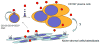
Figure 3. Hedgehog Signaling in Multiple Myeloma
Regulation of MM CSC by the HH signaling pathway. The inhibition of HH signaling (A) inhibits MM CSC displaying the side population phenotype by (B) inducing terminal plasma cells differentiation of MM CSC as indicated by the expression of CD138. (C) Multiple modes of signaling appear to be active in MM. Experimental data suggest that differentiated plasma cells can produce the ligand necessary for CSC survival and proliferation. Blocking signaling leads to CSC differentiation. Normal bone marrow stromal cells can also produce ligand and signal to myeloma cells to support their growth and survival. A possible role for tumor-to-stoma paracrine signaling may also take occur.
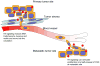
Figure 4. Hh signaling induces EMT and metastasis formation
Cells undergoing EMT under the influence of Hh signaling and become more motile and invasive as they acquire mesenchymal cell properties. This allows cells to escape from the primary tumor and circulate to distant sites. Once established at a distant site, Hh may be required for the clonogenic growth and self-renewal.
Similar articles
- Mechanisms of Hedgehog pathway activation in cancer and implications for therapy.
Scales SJ, de Sauvage FJ. Scales SJ, et al. Trends Pharmacol Sci. 2009 Jun;30(6):303-12. doi: 10.1016/j.tips.2009.03.007. Epub 2009 May 13. Trends Pharmacol Sci. 2009. PMID: 19443052 Review. - Hedgehog signaling pathway and cancer therapeutics: progress to date.
Ruch JM, Kim EJ. Ruch JM, et al. Drugs. 2013 May;73(7):613-23. doi: 10.1007/s40265-013-0045-z. Drugs. 2013. PMID: 23605693 Review. - Targeting Hedgehog signaling and understanding refractory response to treatment with Hedgehog pathway inhibitors.
Queiroz KC, Spek CA, Peppelenbosch MP. Queiroz KC, et al. Drug Resist Updat. 2012 Aug;15(4):211-22. doi: 10.1016/j.drup.2012.05.002. Epub 2012 Aug 19. Drug Resist Updat. 2012. PMID: 22910179 Review. - Molecular pathways: novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells.
Justilien V, Fields AP. Justilien V, et al. Clin Cancer Res. 2015 Feb 1;21(3):505-13. doi: 10.1158/1078-0432.CCR-14-0507. Clin Cancer Res. 2015. PMID: 25646180 Free PMC article. Review. - Hedgehog inhibition as an anti-cancer strategy.
Raju GP, Pham D. Raju GP, et al. Vitam Horm. 2012;88:507-22. doi: 10.1016/B978-0-12-394622-5.00023-7. Vitam Horm. 2012. PMID: 22391319 Review.
Cited by
- Hedgehog signaling pathway and ovarian cancer.
Chen Q, Gao G, Luo S. Chen Q, et al. Chin J Cancer Res. 2013 Jun;25(3):346-53. doi: 10.3978/j.issn.1000-9604.2013.06.04. Chin J Cancer Res. 2013. PMID: 23825912 Free PMC article. - Hedgehog pathway inhibition radiosensitizes non-small cell lung cancers.
Zeng J, Aziz K, Chettiar ST, Aftab BT, Armour M, Gajula R, Gandhi N, Salih T, Herman JM, Wong J, Rudin CM, Tran PT, Hales RK. Zeng J, et al. Int J Radiat Oncol Biol Phys. 2013 May 1;86(1):143-9. doi: 10.1016/j.ijrobp.2012.10.014. Epub 2012 Nov 20. Int J Radiat Oncol Biol Phys. 2013. PMID: 23182391 Free PMC article. - GLI2 regulates TGF-β1 in human CD4+ T cells: implications in cancer and HIV pathogenesis.
Furler RL, Uittenbogaart CH. Furler RL, et al. PLoS One. 2012;7(7):e40874. doi: 10.1371/journal.pone.0040874. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22859956 Free PMC article. - Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA.
Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Niewiadomski P, et al. Cell Signal. 2013 Nov;25(11):2222-30. doi: 10.1016/j.cellsig.2013.07.012. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872071 Free PMC article. - Therapeutic targeting of the p53 pathway in cancer stem cells.
Prabhu VV, Allen JE, Hong B, Zhang S, Cheng H, El-Deiry WS. Prabhu VV, et al. Expert Opin Ther Targets. 2012 Dec;16(12):1161-74. doi: 10.1517/14728222.2012.726985. Epub 2012 Sep 24. Expert Opin Ther Targets. 2012. PMID: 22998602 Free PMC article. Review.
References
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. - PubMed
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. - PubMed
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. - PubMed
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic Hedgehog. Nature. 2005;437:894–7. - PubMed
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23CA107040/CA/NCI NIH HHS/United States
- R01 CA127574-03/CA/NCI NIH HHS/United States
- P01 CA015396/CA/NCI NIH HHS/United States
- R01 CA127574/CA/NCI NIH HHS/United States
- K23 CA107040/CA/NCI NIH HHS/United States
- R01 CA127574-05/CA/NCI NIH HHS/United States
- R01 CA127574-02/CA/NCI NIH HHS/United States
- P01CA15396/CA/NCI NIH HHS/United States
- R01CA127574/CA/NCI NIH HHS/United States
- R01 CA127574-02S1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources