Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain - PubMed (original) (raw)
Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain
Vladimer Darsalia et al. J Cereb Blood Flow Metab. 2011 Jan.
Abstract
Neural stem cells (NSCs) derived from human fetal striatum and transplanted as neurospheres survive in stroke-damaged striatum, migrate from the implantation site, and differentiate into mature neurons. Here, we investigated how various steps of neurogenesis are affected by intrastriatal transplantation of human NSCs at different time points after stroke and with different numbers of cells in each implant. Rats were subjected to middle cerebral artery occlusion and then received intrastriatal transplants of NSCs. Transplantation shortly after stroke (48 hours) resulted in better cell survival than did transplantation 6 weeks after stroke, but the delayed transplantation did not influence the magnitude of migration, neuronal differentiation, and cell proliferation in the grafts. Transplanting greater numbers of grafted NSCs did not result in a greater number of surviving cells or increased neuronal differentiation. A substantial number of activated microglia was observed at 48 hours after the insult in the injured striatum, but reached maximum levels 1 to 6 weeks after stroke. Our findings show that the best survival of grafted human NSCs in stroke-damaged brain requires optimum numbers of cells to be transplanted in the early poststroke phase, before the inflammatory response is established. These findings, therefore, have direct clinical implications.
Figures
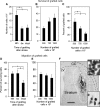
Figure 1
Influence of poststroke delay and cell numbers on the survival and migration of grafted neural stem cells (NSCs). (A and B) Number of surviving cells in NSC grafts at 5 weeks after implantation in stroke-damaged (grafted at 48 hours (A and B) and 6 weeks (A) after the insult) or intact brain. (C) Percentage surviving cells of total number of grafted NSCs at 5 weeks after transplantation performed 48 hours after stroke. (D and E) Migration of grafted NSCs as evaluated outside the transplant core in stroke-damaged or intact brain. Means±s.e.m. *P<0.05, one-way analysis of variance followed by Bonferroni post hoc test. (F) Typical appearance of NSC graft. Solid white line depicts core of the graft; dashed white line marks border of migration. Upper inset—high magnification of the core of the graft; lower inset—high magnification of the area close to the border of the graft. Scale bar=1 mm. CC, corpus callosum; LV, lateral ventricle.
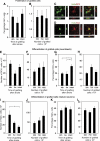
Figure 2
Influence of poststroke delay and cell numbers on the proliferation and differentiation of grafted neural stem cells (NSCs). (A and B) Percentage Ki67+ cells of total number of cells in the graft at 5 weeks after transplantation in the stroke-damaged and intact brain. (C and D) Confocal photomicrographs with orthogonal reconstruction of grafted HuNu+ NSCs coexpressing doublecortin (DCX) and HuD, respectively. Number of DCX+ cells (E and F). Percentage DCX+ cells (G and H) or HuD+ cells (I–L) of total number of migrated, HuNu+ cells in the graft at 5 weeks after implantation in the stroke-damaged or intact brain. Scale bar=15 _μ_m. Means±s.e.m. *P<0.05, one-way analysis of variance followed by Bonferroni post hoc test.
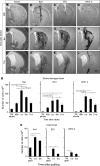
Figure 3
Expression of inflammatory markers at different time points after stroke and transplantation of neural stem cells (NSCs). (A, E, and I) Typical appearance of striatal damage after 30 minutes of middle cerebral artery occlusion in the NeuN-stained sections. Photomicrographs showing immunoreactivity for Iba1 (B, F, and J), ED1 (C, G, and K), and major histocompatibility complex (MHC) class II (D, H, and L) in stroke-damaged brain. (M and N) Time course of number of cells expressing Iba1, ED1, and MHC class II in the stroke-damaged (M) and intact (N) striatum. Animals transplanted at 48 hours (A–D) and 6 weeks (E–L) after stroke were killed 4 hours (A–H) or 5 weeks (I–L) thereafter. All animals in stroke groups had received grafts, and all groups (‘stroke-damaged brain' (M) and ‘intact brain' (N)) were given immunosuppressive treatment. Scale bar=1 mm (F) and 15 _μ_m (insets). Means±s.e.m. *P<0.05, one-way analysis of variance followed by Bonferroni post hoc test.
Similar articles
- Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum.
Darsalia V, Kallur T, Kokaia Z. Darsalia V, et al. Eur J Neurosci. 2007 Aug;26(3):605-14. doi: 10.1111/j.1460-9568.2007.05702.x. Eur J Neurosci. 2007. PMID: 17686040 - Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion.
Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF. Yuan T, et al. Stem Cell Res Ther. 2013 Jun 14;4(3):73. doi: 10.1186/scrt224. Stem Cell Res Ther. 2013. PMID: 23769173 Free PMC article. - Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats.
Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O. Mine Y, et al. Neurobiol Dis. 2013 Apr;52:191-203. doi: 10.1016/j.nbd.2012.12.006. Epub 2012 Dec 28. Neurobiol Dis. 2013. PMID: 23276704 - Neural stem cells: properties and therapeutic potentials for hypoxic-ischemic brain injury in newborn infants.
Lee IS, Jung K, Kim M, Park KI. Lee IS, et al. Pediatr Int. 2010 Dec;52(6):855-65. doi: 10.1111/j.1442-200X.2010.03266.x. Pediatr Int. 2010. PMID: 21029253 Review. - Regulation of Adult Mammalian Neural Stem Cells and Neurogenesis by Cell Extrinsic and Intrinsic Factors.
Matsubara S, Matsuda T, Nakashima K. Matsubara S, et al. Cells. 2021 May 10;10(5):1145. doi: 10.3390/cells10051145. Cells. 2021. PMID: 34068607 Free PMC article. Review.
Cited by
- The potential benefit of stem cell therapy after stroke: an update.
Banerjee S, Williamson DA, Habib N, Chataway J. Banerjee S, et al. Vasc Health Risk Manag. 2012;8:569-80. doi: 10.2147/VHRM.S25745. Epub 2012 Oct 10. Vasc Health Risk Manag. 2012. PMID: 23091389 Free PMC article. Review. - Tanshinone IIA-Loaded Nanoparticle and Neural Stem Cell Therapy Enhances Recovery in a Pig Ischemic Stroke Model.
Kaiser EE, Waters ES, Yang X, Fagan MM, Scheulin KM, Sneed SE, Cheek SR, Jeon JH, Shin SK, Kinder HA, Kumar A, Platt SR, Duberstein KJ, Park HJ, Xie J, West FD. Kaiser EE, et al. Stem Cells Transl Med. 2022 Oct 21;11(10):1061-1071. doi: 10.1093/stcltm/szac062. Stem Cells Transl Med. 2022. PMID: 36124817 Free PMC article. - Defining and designing polymers and hydrogels for neural tissue engineering.
Aurand ER, Lampe KJ, Bjugstad KB. Aurand ER, et al. Neurosci Res. 2012 Mar;72(3):199-213. doi: 10.1016/j.neures.2011.12.005. Epub 2011 Dec 17. Neurosci Res. 2012. PMID: 22192467 Free PMC article. Review. - Regeneration-associated cell transplantation contributes to tissue recovery in mice with acute ischemic stroke.
Nakayama T, Nagata E, Masuda H, Asahara T, Takizawa S. Nakayama T, et al. PLoS One. 2019 Jan 25;14(1):e0210198. doi: 10.1371/journal.pone.0210198. eCollection 2019. PLoS One. 2019. PMID: 30682162 Free PMC article. - Human neural stem cells improve early stage stroke outcome in delayed tissue plasminogen activator-treated aged stroke brains.
Boese AC, Eckert A, Hamblin MH, Lee JP. Boese AC, et al. Exp Neurol. 2020 Jul;329:113275. doi: 10.1016/j.expneurol.2020.113275. Epub 2020 Mar 5. Exp Neurol. 2020. PMID: 32147438 Free PMC article.
References
- Bacigaluppi M, Pluchino S, Jametti LP, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. - PubMed
- Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. - PubMed
- Bliss TM, Kelly S, Shah AK, Foo WC, Kohli P, Stokes C, Sun GH, Ma M, Masel J, Kleppner SR, Schallert T, Palmer T, Steinberg GK. Transplantation of hNT neurons into the ischemic cortex: cell survival and effect on sensorimotor behavior. J Neurosci Res. 2006;83:1004–1014. - PubMed
- Bohl D, Liu S, Blanchard S, Hocquemiller M, Haase G, Heard JM. Directed evolution of motor neurons from genetically engineered neural precursors. Stem Cells. 2008;26:2564–2575. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical