Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity - PubMed (original) (raw)
Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity
Marina Cella et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Natural killer-22 (NK-22) cells are a human NK cell subset situated in mucosal-associated lymphoid tissues that specialize in IL-22 secretion in response to IL-23. Here we investigated the cytokine requirements for NK-22 cell expansion. IL-7 maintained the survival of NK-22 cells and IL-22 production in response to IL-23 but was insufficient to induce robust expansion. Proliferation of NK-22 cells was increased markedly by adding either IL-1beta or IL-2 to IL-7 and was even stronger in the presence of IL-1beta plus IL-2. In contrast to IL-7, continuous culture in IL-1beta and IL-2 modified NK-22 cytokine profiles. IL-1beta promoted constitutive IL-22 secretion rather than acute IL-22 production in response to IL-23 and induced IL-17 in some cells. IL-2 reduced secretion of IL-22 and IL-17, increasing production of IFN-gamma and leukemia inhibitory factor. Functional deviation toward IFN-gamma production also was induced by continuous culture in IL-23. These results demonstrate the functional plasticity of NK-22 cells, which may allow flexible responses to different pathogens. Finally, we found that NK-22 cells released the B-cell survival factor, B-cell activating factor belonging to the TNF family (BAFF), suggesting a potential role of NK-22 cells in promoting B-cell-mediated mucosal immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
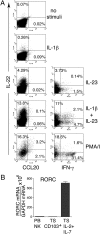
Impact of IL-7 and IL-2 on proliferation and phenotype of NK-22 cells. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-7 alone or in IL-2 plus IL-7. After 7 days of culture cells were analyzed for cell numbers (A). After 10–15 days of culture, we measured cell surface expression of CD56, NKp44, and CCR6 (B); NKp46, CD161, CD117, and CD96 (C); CD33, CD164, CD146, and CD147 (D); CD94, NKG2D, CD16, and 2B4 (E); the receptors for IL-1 (CD121a) and IFN-γ (CD119) (F); and RANKL (presented in two-color analysis versus NKp44) (G). Empty and filled profiles represent staining with control and specific antibodies, respectively. Data shown here were obtained from one tonsil specimen representative of three.
Fig. 2.
NK-22 cells cultured in IL-7 maintain their capacity to produce IL-22 in response to IL-23. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-7 for 10–15 days. Cells were left untreated or stimulated with IL-1β, IL-23, or both and were analyzed for (A) frequencies of IL-22–, CCL20-, and IFN-γ–producing cells (by intracellular staining) and (B) release of IL-22 in the supernatants 18 h after stimulation (by ELISA). Data presented here were obtained from one tonsil specimen representative of three. (C) For comparison, we measured intracellular IL-22 and CCL20 contents of peripheral blood NK cells in response to IL-1β, IL-23, or both.
Fig. 3.
NK-22 cells cultured in IL-2 plus IL-7 exhibit limited IL-22 production in response to IL-23 but produce IFN-γ. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-2 plus IL-7 for 10–15 days. Cells were stimulated with IL-1β, IL-23, IL-1β plus IL-23, or PMA/ionomycin (PMA/I) and were analyzed for (A) production of IL-22, CCL20, and IFN-γ by intracellular staining and (B) expression of RORC mRNA by RT-PCR analysis. Data presented in A were obtained from the same tonsil specimen used for Figs. 2_A_ and 4 D and E to facilitate comparisons. PB NK, peripheral blood NK cells; TS, tonsil.
Fig. 4.
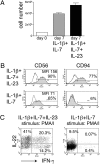
IL-1β plus IL-7 induces proliferation of NK-22 cells and constitutive production of IL-22. Identical numbers of CD56+NKp44+CCR6+CD103− cells sorted from tonsils were cultured in IL-7, IL-1β plus IL-7, or IL-2+IL-7. After 10 days of culture we analyzed (A) cell numbers; (B) expression of CD121a and CD56; (C) release of IL-22 in cell-culture supernatants by ELISA; (D) intracellular IL-22, IFN-γ, and CCL20 after stimulation with IL-1β, IL-23, IL-1β plus IL-23, and PMA/I by flow cytometry; (E) intracellular IFN-γ after stimulation with IL-12; (F) RORC mRNA by RT-PCR. Empty and filled profiles in B represent staining with control and specific antibodies, respectively. Data presented in D and E were obtained from the same tonsil specimen used for Figs. 2_A_ and 3_A_ to facilitate comparisons. PB NK, peripheral blood NK cells; TS, tonsil.
Fig. 5.
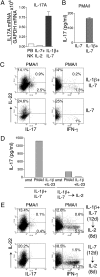
NK-22 cells produce IL-17, IL-22, or IFN-γ depending on the cytokine milieu. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-1β plus IL-7 or in IL-7 alone for 12 days. Cells then were divided into two aliquots: One aliquot was cultured in the original medium; IL-2 was added to the culture medium of the second aliquot. Both cultures were continued for 8 additional days. Cells were analyzed for (A) expression of IL-17 mRNA (by RT-PCR); (B and D) release of IL-17 in culture supernatants 8 h after stimulation with PMA/I or IL-1β plus IL-23 (by ELISA); (C and E) intracellular content of IL-17, IL-22, and IFN-γ after stimulation with PMA/I (by flow cytometry). Data presented here were obtained from one tonsil specimen representative of three. PB NK, peripheral blood NK cells.
Fig. 6.
Addition of IL-23 to IL-1β and IL-7 in the culture medium enhances NK-22 production of IL-22 and IFN-γ. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-1β plus IL-7 or in IL-1β plus IL-7 plus IL-23 for 10 days. Cultures were analyzed for (A) cell numbers; (B) expression of CD56 and CD94; and (C) intracellular IL-2 and IFN-γ after stimulation with PMA/I. Empty and filled profiles in B represent staining with control and specific antibodies, respectively. Data shown here were obtained from one tonsil specimen representative of three.
Fig. 7.
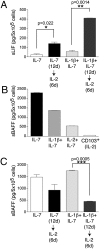
Cultured NK-22 cells release LIF and BAFF. (A) CD56+NKp44+CCR6+CD103− cells were initially cultured in IL-7 or in IL-1β plus IL-7 for 12 days. Cells were divided into two aliquots: One was cultured in the original medium; IL-2 was added to the second aliquot. After 6 additional days of culture, LIF was quantified in the supernatants by ELISA. (B) Release of soluble BAFF by NK-22 cells in different culture conditions. Supernatants were tested 3 days after the last change of medium by ELISA. Note that CD103+ tonsil NK cells do not produce detectable amounts of BAFF. (C) Cells were cultured as described in A, and release of soluble BAFF in supernatants was quantified by ELISA. Data presented here were obtained from one tonsil specimen representative of three.
Similar articles
- A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity.
Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. Cella M, et al. Nature. 2009 Feb 5;457(7230):722-5. doi: 10.1038/nature07537. Epub 2008 Nov 2. Nature. 2009. PMID: 18978771 Free PMC article. - The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors.
de Rham C, Ferrari-Lacraz S, Jendly S, Schneiter G, Dayer JM, Villard J. de Rham C, et al. Arthritis Res Ther. 2007;9(6):R125. doi: 10.1186/ar2336. Arthritis Res Ther. 2007. PMID: 18053164 Free PMC article. - Interleukin-1beta costimulates interferon-gamma production by human natural killer cells.
Cooper MA, Fehniger TA, Ponnappan A, Mehta V, Wewers MD, Caligiuri MA. Cooper MA, et al. Eur J Immunol. 2001 Mar;31(3):792-801. doi: 10.1002/1521-4141(200103)31:3<792::aid-immu792>3.0.co;2-u. Eur J Immunol. 2001. PMID: 11241284 - Functional analyses of cord blood natural killer cells and T cells: a distinctive interleukin-18 response.
Nomura A, Takada H, Jin CH, Tanaka T, Ohga S, Hara T. Nomura A, et al. Exp Hematol. 2001 Oct;29(10):1169-76. doi: 10.1016/s0301-472x(01)00689-0. Exp Hematol. 2001. PMID: 11602318 Review. - NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity.
Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. Albertsson PA, et al. Trends Immunol. 2003 Nov;24(11):603-9. doi: 10.1016/j.it.2003.09.007. Trends Immunol. 2003. PMID: 14596885 Review.
Cited by
- Cytokine-induced cytokine production by conventional and innate lymphoid cells.
Guo L, Junttila IS, Paul WE. Guo L, et al. Trends Immunol. 2012 Dec;33(12):598-606. doi: 10.1016/j.it.2012.07.006. Epub 2012 Sep 5. Trends Immunol. 2012. PMID: 22959641 Free PMC article. Review. - Group 3 innate lymphoid cells require BATF to regulate gut homeostasis in mice.
Wu X, Khatun A, Kasmani MY, Chen Y, Zheng S, Atkinson S, Nguyen C, Burns R, Taparowsky EJ, Salzman NH, Hand TW, Cui W. Wu X, et al. J Exp Med. 2022 Nov 7;219(11):e20211861. doi: 10.1084/jem.20211861. Epub 2022 Sep 1. J Exp Med. 2022. PMID: 36048018 Free PMC article. - Homing receptor expression is deviated on CD56+ blood lymphocytes during pregnancy in Type 1 diabetic women.
Burke SD, Seaward AV, Ramshaw H, Smith GN, Virani S, Croy BA, Lima PD. Burke SD, et al. PLoS One. 2015 Mar 20;10(3):e0119526. doi: 10.1371/journal.pone.0119526. eCollection 2015. PLoS One. 2015. PMID: 25793768 Free PMC article. - Metabolic mediators: microbial-derived metabolites as key regulators of anti-tumor immunity, immunotherapy, and chemotherapy.
Lu S, Wang C, Ma J, Wang Y. Lu S, et al. Front Immunol. 2024 Sep 16;15:1456030. doi: 10.3389/fimmu.2024.1456030. eCollection 2024. Front Immunol. 2024. PMID: 39351241 Free PMC article. Review. - Transcriptional control of ILC identity.
Korchagina AA, Shein SA, Koroleva E, Tumanov AV. Korchagina AA, et al. Front Immunol. 2023 Mar 9;14:1146077. doi: 10.3389/fimmu.2023.1146077. eCollection 2023. Front Immunol. 2023. PMID: 36969171 Free PMC article. Review.
References
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. - PubMed
- Aujla SJ, Kolls JK. IL-22: A critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. - PubMed
- Zenewicz LA, Flavell RA. IL-22 and inflammation: Leukin’ through a glass onion. Eur J Immunol. 2008;38:3265–3268. - PubMed
- Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources