Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus - PubMed (original) (raw)
Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus
Yvette I Sheline et al. Proc Natl Acad Sci U S A. 2010.
Abstract
To better understand intrinsic brain connections in major depression, we used a neuroimaging technique that measures resting state functional connectivity using functional MRI (fMRI). Three different brain networks--the cognitive control network, default mode network, and affective network--were investigated. Compared with controls, in depressed subjects each of these three networks had increased connectivity to the same bilateral dorsal medial prefrontal cortex region, an area that we term the dorsal nexus. The dorsal nexus demonstrated dramatically increased depression-associated fMRI connectivity with large portions of each of the three networks. The discovery that these regions are linked together through the dorsal nexus provides a potential mechanism to explain how symptoms of major depression thought to arise in distinct networks--decreased ability to focus on cognitive tasks, rumination, excessive self-focus, increased vigilance, and emotional, visceral, and autonomic dysregulation--could occur concurrently and behave synergistically. It suggests that the newly identified dorsal nexus plays a critical role in depressive symptomatology, in effect "hot wiring" networks together; it further suggests that reducing increased connectivity of the dorsal nexus presents a potential therapeutic target.
Conflict of interest statement
Conflict of interest statement: Y.I.S. has served on the advisory board and speakers’ bureau of Eli Lilly, Inc. M.A.M. serves as a consultant for Avid Radiopharmaceuticals. Y.I.S. is independent of any commercial provider, had full access to all of the data in this study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. No author named on the title page of this study has any financial interest in the results of the study or any other conflict of interest relevant to the subject matter of this manuscript.
Figures
Fig. 1.
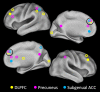
Seed regions and targets. Each of the three solid circles corresponds to a seed region in the DLPFC, part of the CCN (yellow); precuneus, part of the DMN (pink); and subgenual and pregenual cingulate cortex, the affective division of the ACC (16) (turquoise). The correspondingly colored open circles represent regions with significantly increased connectivity with the respective seed regions.
Fig. 2.
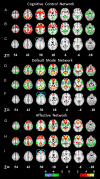
Comparison of connectivity maps for depressed and control subjects across three networks. Connectivity of the CCN for control participants (A), depressed participants (B), and between-group differences (C); connectivity of DMN for controls (D), depressed participants (E), and between-group differences (F); and connectivity of the AN for controls (G), depressed participants (H), and between-group differences (I). Networks were identified by seed regions placed in the DLPFC (A_–_C), precuneus (D_–_F), and subgenual ACC (G_–_I). In each network, the dorsal nexus is seen at z = 30. The color bar indicates that images were thresholded at z = 2.58, P < 0.01.
Fig. 3.
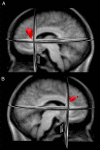
A 3D image of the dorsal nexus. (A) Left (−24, 35, 28). (B) Right (18, 34, 29). The nexus was created from a conjunction analysis of the overlap of the areas of significantly increased connectivity among the three networks arising from seeds placed in the DLPFC, precuneus, and subgenual ACC. The individual connectivity maps for these seed regions are shown in Fig. 2. The nexus is comprised primarily of medial BA9, a portion of the ACC (BA32), and a small portion of medial BA8.
Fig. 4.
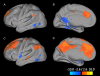
Connectivity map from the dorsal nexus to all of the voxels in the brain. Pictured are lateral and medial surface connectivity of the left hemisphere for both control (A and B) and depressed (C and D) participants. Dramatically higher connectivity for depressed participants is evident.
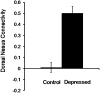
Fig. 5.
Comparison of depressed and control participants for mean resting-state connectivity between the dorsal nexus and the combined three seed regions in the DLPFC, precuneus, and subgenual ACC. Images were thresholded at z = 2.58, P < 0.01, as shown in Figure 4.
Similar articles
- Pre-scan cortisol is differentially associated with enhanced connectivity to the cognitive control network in young adults with a history of depression.
Peters AT, Jenkins LM, Stange JP, Bessette KL, Skerrett KA, Kling LR, Welsh RC, Milad MR, Phan KL, Langenecker SA. Peters AT, et al. Psychoneuroendocrinology. 2019 Jun;104:219-227. doi: 10.1016/j.psyneuen.2019.03.007. Epub 2019 Mar 12. Psychoneuroendocrinology. 2019. PMID: 30889471 Free PMC article. - Gamma-hydroxybutyrate increases brain resting-state functional connectivity of the salience network and dorsal nexus in humans.
Bosch OG, Esposito F, Dornbierer D, Havranek MM, von Rotz R, Kometer M, Staempfli P, Quednow BB, Seifritz E. Bosch OG, et al. Neuroimage. 2018 Jun;173:448-459. doi: 10.1016/j.neuroimage.2018.03.011. Epub 2018 Mar 7. Neuroimage. 2018. PMID: 29524621 Clinical Trial. - Aberrant resting-state functional connectivity in limbic and salience networks in treatment--naïve clinically depressed adolescents.
Pannekoek JN, van der Werff SJ, Meens PH, van den Bulk BG, Jolles DD, Veer IM, van Lang ND, Rombouts SA, van der Wee NJ, Vermeiren RR. Pannekoek JN, et al. J Child Psychol Psychiatry. 2014 Dec;55(12):1317-27. doi: 10.1111/jcpp.12266. Epub 2014 May 15. J Child Psychol Psychiatry. 2014. PMID: 24828372 - Resting-state functional connectivity in major depressive disorder: A review.
Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Mulders PC, et al. Neurosci Biobehav Rev. 2015 Sep;56:330-44. doi: 10.1016/j.neubiorev.2015.07.014. Epub 2015 Jul 30. Neurosci Biobehav Rev. 2015. PMID: 26234819 Review. - Mapping cognitive and emotional networks in neurosurgical patients using resting-state functional magnetic resonance imaging.
Catalino MP, Yao S, Green DL, Laws ER, Golby AJ, Tie Y. Catalino MP, et al. Neurosurg Focus. 2020 Feb 1;48(2):E9. doi: 10.3171/2019.11.FOCUS19773. Neurosurg Focus. 2020. PMID: 32006946 Free PMC article. Review.
Cited by
- Altered amygdalar resting-state connectivity in depression is explained by both genes and environment.
Córdova-Palomera A, Tornador C, Falcón C, Bargalló N, Nenadic I, Deco G, Fañanás L. Córdova-Palomera A, et al. Hum Brain Mapp. 2015 Oct;36(10):3761-76. doi: 10.1002/hbm.22876. Epub 2015 Jun 19. Hum Brain Mapp. 2015. PMID: 26096943 Free PMC article. - Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches.
Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T, Trivedi MH. Phillips ML, et al. Am J Psychiatry. 2015 Feb 1;172(2):124-38. doi: 10.1176/appi.ajp.2014.14010076. Am J Psychiatry. 2015. PMID: 25640931 Free PMC article. Review. - Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design.
Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, Oquendo MA, Bruder G, Pizzagalli D, Toups M, Cooper C, Adams P, Weyandt S, Morris DW, Grannemann BD, Ogden RT, Buckner R, McInnis M, Kraemer HC, Petkova E, Carmody TJ, Weissman MM. Trivedi MH, et al. J Psychiatr Res. 2016 Jul;78:11-23. doi: 10.1016/j.jpsychires.2016.03.001. Epub 2016 Mar 15. J Psychiatr Res. 2016. PMID: 27038550 Free PMC article. Clinical Trial. - Nitrous Oxide Alters Functional Connectivity in Medial Limbic Structures in Treatment-Resistant Major Depression.
Conway CR, Palanca BJA, Zeffiro T, Gott BM, Brown F, de Leon V, Barnes L, Nguyen T, Xiong W, Lessov-Schlaggar CN, Espejo G, Mennerick S, Zorumski CF, Nagele P. Conway CR, et al. medRxiv [Preprint]. 2024 Aug 17:2024.08.12.24311729. doi: 10.1101/2024.08.12.24311729. medRxiv. 2024. PMID: 39185528 Free PMC article. Preprint. - Default mode network connectivity encodes clinical pain: an arterial spin labeling study.
Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Loggia ML, et al. Pain. 2013 Jan;154(1):24-33. doi: 10.1016/j.pain.2012.07.029. Epub 2012 Oct 27. Pain. 2013. PMID: 23111164 Free PMC article.
References
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. - PubMed
- Rogers MA, et al. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neurosci Res. 2004;50:1–11. - PubMed
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. - PubMed
- Fitzgerald PB, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. - PubMed
- Siegle G, Thompson W, Carter C, Steinhauser S, Thase M. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry. 2007;61:198–209. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources